Need muscle for a tough spot? Turn to fat stem cells
(PhysOrg.com) -- Stem cells derived from fat have a surprising trick up their sleeves: Encouraged to develop on a stiff surface, they undergo a remarkable transformation toward becoming mature muscle cells. The new research appears in the journal Biomaterials. The new cells remain intact and fused together even when transferred to an extremely stiff, bone-like surface, which has University of California, San Diego bioengineering professor Adam Engler and colleagues intrigued. These cells, they suggest, could hint at new therapeutic possibilities for muscular dystrophy.
In diseases like muscular dystrophy or a heart attack, “muscle begins to die and undergoes its normal wounding processes,” said Engler, a bioengineering professor at the Jacobs School of Engineering at UC San Diego. “This damaged tissue is fundamentally different from a mechanical perspective” than healthy tissue.
Transplanted stem cells might be able to replace and repair diseased muscle, but up to this point the transplants haven’t been very successful in muscular dystrophy patients, he noted. The cells tend to clump into hard nodules as they struggle to adapt to their new environment of thickened and damaged tissue.
Engler, postdoctoral scholar Yu Suk Choi and the rest of the team think their fat-derived stem cells might have a better chance for this kind of therapy, since the cells seem to thrive on a stiff and unyielding surface that mimics the damaged tissue found in people with MD.
In their study in the journal Biomaterials, the researchers compared the development of bone marrow stem cells and fat-derived stem cells grown on surfaces of varying stiffness, ranging from the softness of brain tissue to the hardness of bone.
Cells from the fat lineage were 40 to 50 times better than their bone marrow counterparts at displaying the proper proteins involved in becoming muscle. These proteins are also more likely to “turn on” in the correct sequence in the fat-derived cells, Engler said.
Subtle differences in how these two types of cells interact with their environment are critical to their development, the scientists suggest. The fat-derived cells seem to sense their “niche” on the surfaces more completely and quickly than marrow-derived cells. “They are actively feeling their environment soon, which allows them to interpret the signals from the interaction of cell and environment that guide development,” Choi explained.
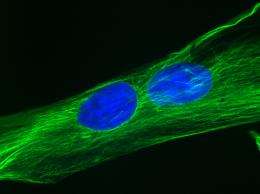
Perhaps most surprisingly, muscle cells grown from the fat stem cells fused together, forming myotubes to a degree never previously observed. Myotubes are a critical step in muscle development, and it’s a step forward that Engler and colleagues hadn’t seen before in the lab.
The fused cells stayed fused when they were transferred to a very stiff surface. “These programmed cells are mature enough so that they don’t respond the environmental cues” in the new environment that might cause them to split apart, Engler says.
Engler and colleagues will now test how these new fused cells perform in mice with a version of muscular dystrophy. The cells survive in an environment of stiff tissue, but Engler cautions that there are other aspects of diseased tissue such as its shape and chemical composition to consider. “From the perspective of translating this into a clinically viable therapy, we want to know what components of the environment provide the most important cues for these cells,” he said.
Provided by University of California - San Diego