This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Probing the depths of complex electron shells: New insights into uranium's tricky chemistry
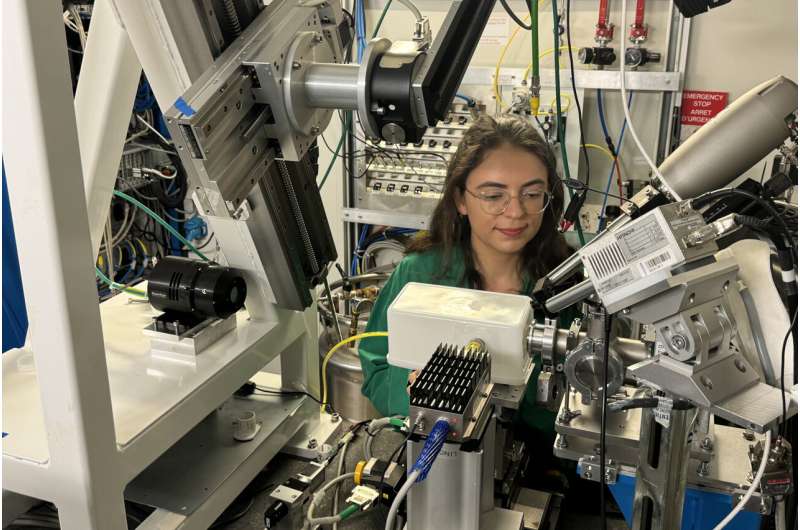
The heavy metal uranium is, besides its radioactive reputation, known for its intricate chemistry and diverse bonding behaviors. Now, an international team of scientists have utilized synchrotron light at the Rossendorf Beamline (ROBL) to explore the unique properties of low-valent uranium compounds, as the researchers report in the journal Nature Communications.
At the European Synchrotron Radiation Facility (ESRF) in Grenoble, the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) runs four experimental stations for radiochemical experiments.
Uranium, belonging to the periodic table's actinide elements, has long intrigued scientists due to its complex electron configurations. Its unusual and diverse bonding characteristic manifests itself in a variety of oxidation states—at times, even exotic.
"In our current study we were focused on low-valent uranium, which contains more electrons in its inner shells compared to other, more common uranium compounds. Specifically, we investigated the behavior of uranium's so-called 5f electrons—electrons that, despite being located in the inner shells, play a crucial role in the element's chemical properties. These electrons significantly influence how uranium bonds with other elements," says Ph.D. student Clara Silva from HZDR's Institute of Resource Ecology.
"Due to the radioactive nature of uranium, the experiments were carried out here, a facility specifically designed for actinide research. This environment provided the necessary safety protocols and advanced equipment to conduct the study," says Prof. Kristina Kvashnina, head of ROBL and the Institute's Department Molecular Structure.
To gain the new insights, the team used a technique called resonant inelastic X-ray scattering known as RIXS. RIXS is a powerful method that involves bombarding a material with X-rays and then measuring the energy lost as the X-rays scatter off the material. This energy loss provides detailed information about the electronic structure of the material, helping scientists understand how electrons, like those in the 5f orbital of uranium, behave and interact.
The researchers supplemented their findings with another specialized X-ray technique: the so-called HERFD-XANES method provides highly detailed information about the electronic structure of materials by combining high-energy resolution fluorescence detection—the HERFD part—with X-ray absorption near edge structure analysis, abbreviated as XANES.
Understanding uranium's unique bonding behavior
"For the first time, we were able to accurately identify and directly detect the three-valent oxidation state in uranium, or U(III) in short, revealing how uranium atoms interact and bond with elements such as fluorine and chlorine," Kvashnina outlines the results of her group's work which was 15 years in the making.
The findings shed new light onto the nature of actinide bonding and demonstrate how uranium's 5f electrons respond to changes in their environment.
Studying low-valent uranium compounds is not without its challenges. These compounds are less stable than other uranium-containing materials, requiring carefully controlled conditions to prevent unwanted reactions.
To maintain the stability of the uranium samples, the research was conducted under anoxic conditions—meaning no exposure to oxygen—and at extremely low temperatures. Additionally, the complexity of the data required the use of cutting-edge theoretical methods to accurately model the electronic structure and bonding properties of uranium.
Unexpected insights and broader implications
"One of the most surprising findings of the study was the degree of sensitivity of uranium's 5f electrons to their local environment, which affects the ionic character of its bonds. This discovery challenges existing theories about actinide bonding and opens up new avenues of research in actinide physics and chemistry," Silva says.
And, besides being of its fundamental importance, the team´s work is also interesting in practical terms, for instance, both for general aspects of radiation protection and for the safety of radioactive waste repositories. Low-valent uranium compounds are particularly notable for their low solubility, which reduces their environmental mobility and helps contain contamination.
Furthermore, the implications of this research could be far-reaching even beyond this point. By enhancing our understanding of low-valent uranium systems, scientists can now improve theoretical models that predict the behavior of such complex elements.
These insights will aid future research across various scientific disciplines, potentially leading to new developments in fields ranging from nuclear science to environmental chemistry.
More information: C. L. Silva et al, On the origin of low-valent uranium oxidation state, Nature Communications (2024). DOI: 10.1038/s41467-024-50924-7
Journal information: Nature Communications
Provided by Helmholtz Association of German Research Centres