This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New filter removes chemical contaminants from water even at very low concentrations
Pharmaceuticals and personal care products pose a major environmental threat. These chemicals, found in everyday items like medicines and cosmetics, can pollute waterways, harming the plants and animals living in the waterways and the humans who use the water.
The pollutants are often present at very low concentrations, and existing filtration methods aren't always selective enough to remove the chemicals that cause environmental damage.
A team of researchers from Japan and the United States, led by Professor Shuhei Furukawa from the Institute for Integrated Cell-Material Sciences (WPI-iCeMS) at Kyoto University, have developed a new method for removing these chemicals from water.
"Current workflows for water treatment involve steps for pollutant detection and pollutant removal, and the two steps are normally conducted separately through different materials or industrial set-up," explains Professor Furukawa. "We developed new membrane materials that can simultaneously detect and remove trace-level pollutants."
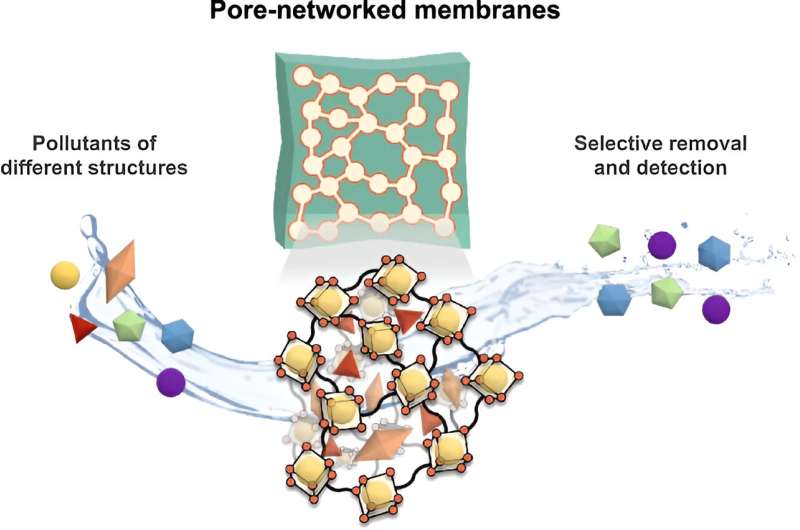
The new approach uses a polymer membrane that houses an interconnected network of pores constructed from metal-organic polyhedra, which are like tiny cages to filter and capture the targeted chemical molecules.
The design of the pores is critical to the effectiveness of the membrane. The chemicals in pharmaceuticals and personal care products are relatively large, and most current adsorbents have pores that are too small to capture these molecules.
The researchers tested the pore-networked membrane against 13 different pharmaceuticals and personal care products at different concentrations, and they compared its performance to a variety of existing filtration systems.
They found that the new membrane was better at filtering out these chemicals than existing systems, and it could also be refined to selectively adsorb particular pharmaceutical products, even at incredibly low concentrations.
The findings are published in the journal Communications Materials.
"An optimized pore-networked membrane detected and removed target drugs below the parts-per-billion level in real water samples, demonstrating the potential for its use in real water-treatment workflows," says Dr. Idaira Pacheco-Fernández, an environmental scientist from WPI-iCeMS, Kyoto University, who contributed to this project for water analysis.
The new membrane is also designed so the captured molecules can be extracted into solution for testing, enabling real-time monitoring of contamination.
The next step for the researchers is to vary the pore-networked membrane design by using other types of porous fillers which could filter out different types and sizes of molecules. They're also interested in whether this approach could capture and detect small molecules from other liquids, such as blood.
More information: Zaoming Wang et al, Pore-networked membrane using linked metal-organic polyhedra for trace-level pollutant removal and detection in environmental water, Communications Materials (2024). DOI: 10.1038/s43246-024-00607-z
Journal information: Communications Materials
Provided by Kyoto University