This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists reveal molecular link between glucose sensing and pyroptosis cell death
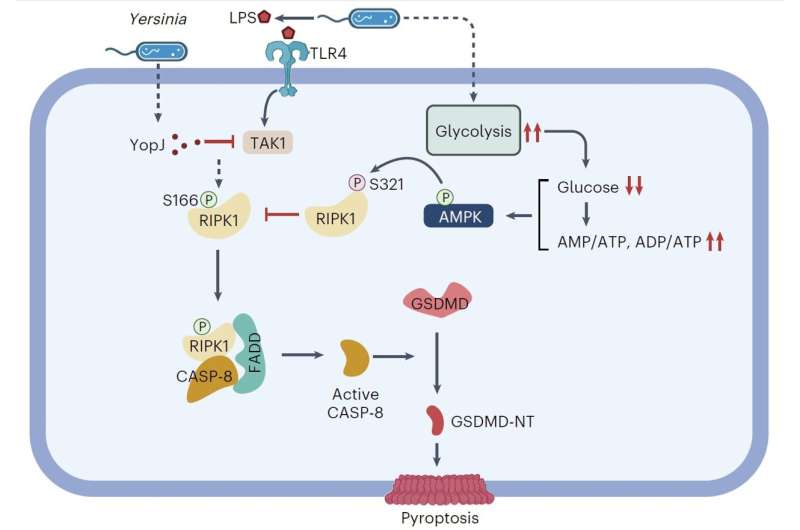
According to a study published in Nature Microbiology on June 6, researchers led by Prof. Xu Daichao from the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences have uncovered the molecular link between glucose sensing and non-classical pyroptosis.
Nutritional status and pyroptosis play a critical role in host defense against pathogens. Accumulating evidence suggests that changes in the nutritional status of host cells, particularly glucose levels, are critical for their response to pathogen infection.
Host cell glucose levels may fluctuate during bacterial infection. This fluctuation may be part of the host's immune defense and therefore beneficial, or it may also be an inevitable adverse consequence of infection.
Pyroptosis is a regulated form of cell death that induces an inflammatory response in host cells and contributes to the host cell's immune defense. However, the molecular link between host cell nutritional status and pyroptosis during microbial infection remains unclear.
AMPK is an intracellular serine/threonine kinase and a central energy sensor that responds to metabolic stress. It can be activated not only by sensing a decrease in intracellular energy status, but also by sensing glucose levels.
During microbial infection, host cells require large amounts of energy to drive the immune response. Therefore, most intracellular pathogen infections are associated with the activation of host AMPK.
In this study, the researchers found that in the early stages of Yersinia infection, Yersinia can induce a sharp increase in glycolysis levels in host immune cells, leading to a rapid and significant decrease in glucose levels within immune cells and blood glucose levels in mice, and ultimately overactivation of host AMPK.
Under normal conditions, host cells activate the RIPK1-caspase-8-GSDMD-mediated non-classical pyroptosis pathway upon Yersinia infection to restrict their proliferation. However, AMPK activated in host cells can directly inhibit RIPK1 through an inhibitory phosphorylation at the highly conserved S321 site, thereby limiting its kinase activity and subsequent caspase-8 activation, thus preventing pyroptosis, and promoting unfavorable responses to infection.
The researchers found that overactivating AMPK in mice, either by using AMPK agonists or methods such as glucose depletion during Yersinia infection, can worsen the severity of the infection. Conversely, knocking out AMPK in macrophages or supplementing glucose to inhibit AMPK activity during Yersinia infection in mice can significantly improve the resistance of mice to Yersinia infection.
In conclusion, this study investigated the impact of alterations in glucose homeostasis on the regulation of AMPK activation and RIPK1-dependent pyroptosis during Yersinia infection.
Additionally, the regulatory effect of pathogen infection on alterations in host cell nutritional status and its impact on host cell immune defense was elucidated. The results of this study suggest that maintaining patients' nutritional status and blood glucose levels may be crucial in the treatment of Yersinia infection.
More information: Yuanxin Yang et al, Yersinia infection induces glucose depletion and AMPK-dependent inhibition of pyroptosis in mice, Nature Microbiology (2024). DOI: 10.1038/s41564-024-01734-6
Journal information: Nature Microbiology
Provided by Chinese Academy of Sciences