This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A first look inside radium's solid-state chemistry
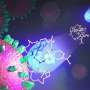
For the first time in history, scientists have measured radium's bonding interactions with oxygen atoms in an organic molecule. Scientists have not measured this bonding before because radium-226 is available only in small amounts and it is highly radioactive (radium is one million times more radioactive than the same mass of uranium), making it challenging to work with.
The findings are published in the journal Nature Chemistry.
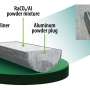
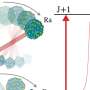
Using oxygen as the donor atom, the researchers developed a way to synthesize and crystallize the radium complex rapidly and on a small scale. Next, they measured the X-ray diffraction pattern of the complex. This pattern is created by the complex's crystal structure and reveals its structure and bonding characteristics.
Certain radium isotopes show promise for targeted alpha therapy treatment for cancers. For this type of treatment, radium must be bonded to another molecule, called a "chelator," and delivered directly to tumors in the body. There, the radium gives off powerful radiation that travels only a very short distance to attack the tumor and leaves surrounding cells unharmed.
When developing these chelators, scientists typically use barium because it is chemically similar to radium. However, this study showed that radium is quite different from barium. The result gives scientists information that may help them use radium in future cancer treatments.
Working with radium, which is highly radioactive, required the researchers to develop a process for synthesis and crystallization on a nanogram scale.
Their success using this technique to characterize radium potentially allows scientists to learn exactly how radium binds to other elements—oxygen or nitrogen, for example. Since nitrogen and oxygen are elements typically present in chelators, and radium interacts with them during bonding, this information will be helpful for developing chelators to carry radium to cancer sites in targeted alpha therapy treatment.
This work also demonstrates significant differences between radium and barium in how they interact with chelators, suggesting that barium is not always a good stand-in for radium when developing these chelators. The methods the researchers used to characterize and analyze radium potentially could be used to learn about other challenging radioactive complexes.
More information: Frankie D. White et al, Structure and bonding of a radium coordination compound in the solid state, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01366-z
Journal information: Nature Chemistry
Provided by US Department of Energy