This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Peptidomimetics open new opportunities in drug discovery
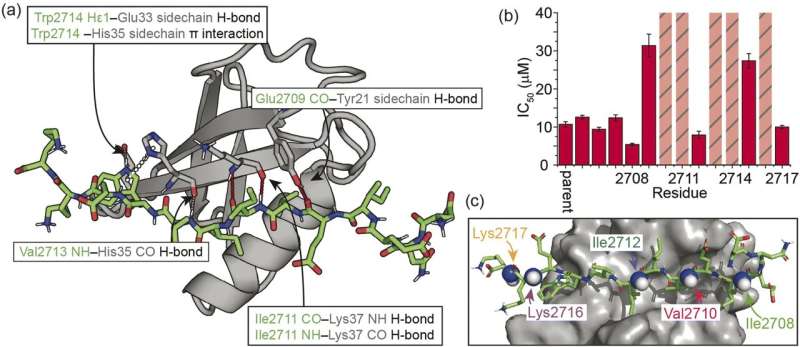
A relatively simple peptide modification could unlock a whole class of targets for drug discovery, new research shows.
Researchers at the University of Birmingham, in collaboration with the Universities of Bristol and Leeds, successfully demonstrated a route by which peptides could be modified to make them promising reagents for disease diagnostics and drug discovery.
Traditional targets in drug discovery are enzymes such as proteases and kinases. These proteins are attractive targets because they have well defined "binding sites" for their substrates—the molecules with which the enzymes interact. It is now relatively straightforward to develop molecules that mimic the substrate and which inhibit or modify the function of the target.
In contrast, interactions between proteins—so called protein-protein interaction (PPIs)—regulate most biological functions, including that of enzymes. PPIs are far more numerous than traditional drug discovery targets, so blocking PPIs potentially opens-up a much wider range of drug targets.
PPIs have traditionally been considered too difficult to use as drug targets, however, because their binding sites are larger, with fewer grooves or pockets to which small molecule compounds can bind. Understanding how PPIs occur and how to control this represents a first step towards drug discovery against these important targets.
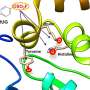
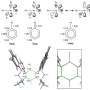
In the new study, published in Chemical Science, the team focused on a PPI that involves β-strand formation at the interface. A β-strand is a specific type of secondary structure that is used to build 3D structure in proteins. By taking a small peptide sequence from the part of the protein where the β-strand forms, and making modifications to its backbone, the team were able to show it binds more quickly and more strongly to the target.
"Our modification uses relatively simple chemistry and has taught us about how peptides bind to their targets in a β-strand conformation and how to control binding," said lead researcher Professor Andy Wilson, "This in turn opens up the path to drug discovery for β-strand mediated PPIs targets."
The team has shown how this could be done for one specific type of PPI—the SIM/SUMO interaction, which in itself plays fundamental roles in protein stability, response to stress and the cell cycle. The next steps will be to demonstrate the approach can be generalized for multiple different PPIs.
More information: Emma E. Cawood et al, Understanding β-strand mediated protein–protein interactions: tuning binding behaviour of intrinsically disordered sequences by backbone modification, Chemical Science (2024). DOI: 10.1039/D4SC02240H
Journal information: Chemical Science
Provided by University of Birmingham