This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New development opens the door to more studies of protein movements
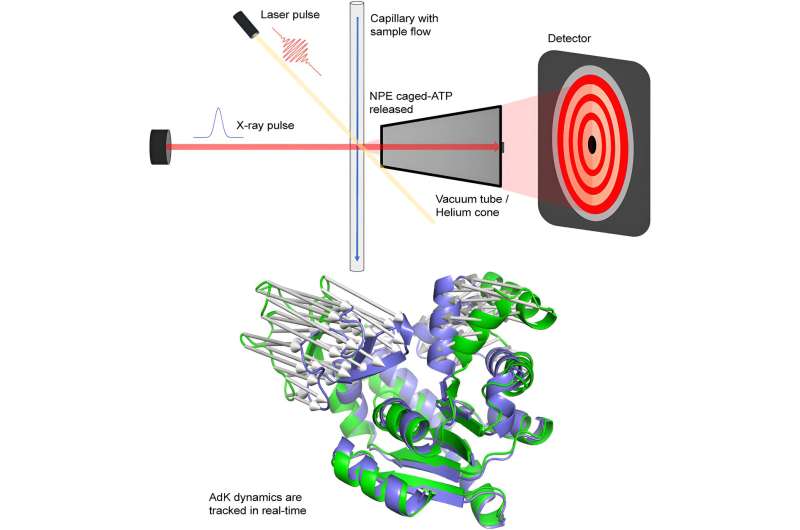
A new way to study protein movements has been developed by researchers at Umeå University and the MAX IV Laboratory in Lund. The method enables significantly more experiments than before and allows us to learn more about vital processes in the cells of humans, animals, and plants. The work is published in the journal Structure.
Proteins must move to perform their biological tasks in the cell. Such movements are called protein dynamics and are encoded in the protein's amino acid sequence through evolution. Since protein dynamics control essential processes like photosynthesis, nerve impulses, and energy conversion, many research groups are engaged in developing methods to study these structural changes at the molecular level.
One way is to use X-ray radiation from a synchrotron. The problem is that there are only a handful of synchrotron stations in the world that specialize in time-resolved experiments, which severely limits researchers' access.
Magnus Andersson's research group at Umeå University, together with a team from the MAX IV Laboratory led by Tomás Plivelic, has developed a method that instead combines fast X-ray detectors with indirect laser activation. This makes it possible to conduct time-resolved experiments at more synchrotron stations, including MAX IV in Sweden.
"Our experiment has great potential to pave way for a series of interesting studies of protein dynamics at the MAX IV synchrotron. For example, we would like to study how lipids in the cell membrane affect the dynamics of transport proteins that influence stress regulation in plants, as well as the heart's pump cycle," says Andersson, Associate Professor at the Department of Chemistry.
What the researchers have done is that instead of using specialized synchrotron stations, they used X-ray detectors that can register X-ray radiation on a micro- to millisecond time scale. These were combined with indirect laser activation of an ATP-dependent protein called adenylate kinase.
The reaction could be followed for 50 milliseconds with minimal negative effects from the X-ray radiation, which can otherwise break down biological material. For activation, an inactive form of ATP—a so-called caged compound—was used, which releases ATP when the laser hits the sample. This method enables studies of dynamics in a large number of proteins.
"An important aspect is that this project connects research in northern Sweden with national infrastructure in the southern parts of the country, which strengthens our research collaboration and enables further progress in the field," says Andersson.
More information: Konstantinos Magkakis et al, Real-time structural characterization of protein response to a caged compound by fast detector readout and high-brilliance synchrotron radiation, Structure (2024). DOI: 10.1016/j.str.2024.05.015
Journal information: Structure
Provided by Umea University