This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Protein structures signal fresh targets for anticancer drugs
Cell replication in our bodies is triggered by a cascade of molecular signals transmitted between proteins. Compounds that block these signals when they run amok show potential as cancer drugs.
Scientists have uncovered the molecular mechanisms that underlie a step in the signal transmission pathway that requires three proteins to link up. The detailed knowledge about this three-protein complex, determined with synchrotron X-ray user facilities, points the way to new targets for drugs that fight certain types of cancer.
Some promising anticancer drugs work by jamming proteins that transmit signals for cells in the body to replicate. This slows the growth of tumors. However, drug-resistance mechanisms enable the signals to bypass the jam.
Scientists working on cancer treatments therefore need to gain a molecular-level understanding of the ways signaling proteins interact with each other.
In a 2022 study published in Nature, scientists used biochemical experiments combined with protein-structure studies to understand the details of a key step in the signaling pathway. The results provide a sharper picture of a process that had remained unclear despite decades of study. This could lead to improved drugs for lung, colorectal, pancreatic, and other cancers.
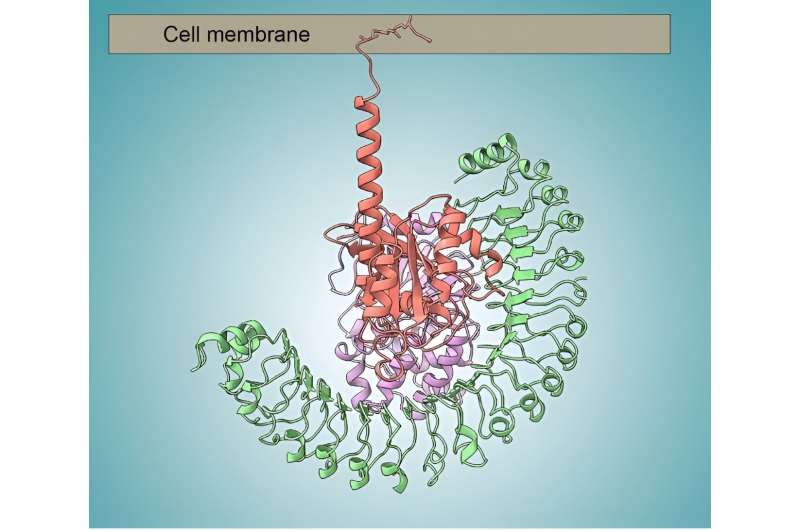
This work focused on one link in the cell-replication signaling chain, involving proteins known as SHOC2, PPIC, and RAS. When assembled, this three-protein complex becomes chemically active, enabling the next step in the signal cascade.
To obtain detailed information about where individual atoms in the proteins are located, the research team used electron microscopy at Genentech and protein crystallography at the Stanford Synchrotron Radiation Laboratory.
To understand how the three proteins fit together like a jigsaw puzzle, the researchers used a technique called small-angle X-ray scattering (SAXS) at the Advanced Light Source, a Department of Energy Office of Science light source user facility at Lawrence Berkeley National Laboratory.
Using the SAXS data, the researchers were able to capture snapshots of the large, flexible protein complex in native form (suspended in solution). This allowed them to model the flexibility of SHOC2, which acts as a scaffold for the other two proteins.
Together with the other structural data, biochemical studies, and computer simulations, the work answered many outstanding questions, including how disease-relevant mutations affect assembly of the complex and how the proteins collectively work to activate the next step in the signaling process. In general, the work establishes fresh avenues for the discovery of new classes of targeted anticancer drugs.
More information: Nicholas P. D. Liau et al, Structural basis for SHOC2 modulation of RAS signalling, Nature (2022). DOI: 10.1038/s41586-022-04838-3
Journal information: Nature
Provided by US Department of Energy