This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New promising catalyst: Study reveals tungsten pentaboride's resistance to sulfur poisoning
A group of researchers led by Professor Alexander Kvashnin from Skoltech's Energy Transition Center has published a new paper on tungsten pentaboride, WB5-x, a substance that has a number of advantages over traditional catalysts.
Scientists have identified and studied the stable surfaces of the WB5-x crystal and revealed that the new catalyst is not poisoned by sulfur-containing impurities, which means it does not lose its activity.
Tungsten pentaboride can potentially be used as a catalyst or a co-catalyst in filters for cleaning industrial exhaust gases, mining precious metals, photocatalytic production of hydrogen, and in other fields. The study is published in Scientific Reports.
Previously, scientists synthesized tungsten pentaboride WB5-x, refined the method for synthesizing superhard tungsten boride powder, in collaboration with Tomsk Polytechnic University, and revealed that the synthesized substance significantly increases the efficiency of reactions to convert carbon dioxide into methane and to produce hydrogen from an aqueous solution of ethanol.
"In the new paper, we found out that the more boron in a compound, the better its catalytic properties. This seemed rather paradoxical to us because, as a rule, the active centers of the catalyst are metal atoms.
"We have the highest tungsten boride, that is, among the compounds of boron and metal it contains the largest amounts of boron, so tungsten pentaboride seems to be a promising catalyst," said leading author of the study, Aleksandra Radina, a Ph.D. student of the Materials Science and Engineering program at Skoltech.
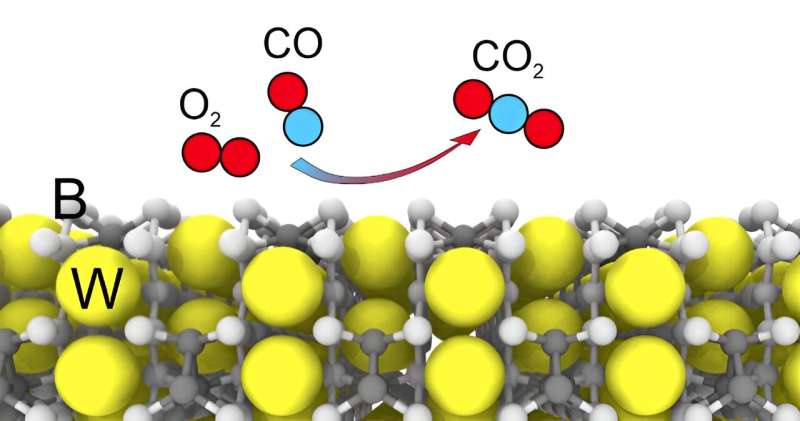
The authors found stable surfaces of the compound: One has mainly boron on top, the other has tungsten atoms. Having compared them, the researchers concluded that boron actively participates in both adsorption and catalysis processes.
The study made another important conclusion: Tungsten pentaboride is not susceptible to poisoning from sulfur-containing compounds. This means that its activity will not be affected by so-called contact poisons, which include substances containing oxygen, sulfur, and metal ions.
According to the authors, poisoning of catalysts is a big problem. For example, oil is purified from any sulfur compounds, but it still contains a small amount of it, so the catalysts will work less time due to their poisoning.
"Catalysts based on noble and rare earth metals are poisoned by sulfur-containing compounds, so it was important for us to consider a large number of gas molecules and their positions on the surface of the material.
"We examined 10 molecules of atmospheric gases, including CO2. According to preliminary data, our catalyst is not poisoned. This is a significant advantage, in addition to the price," Radina said.
More information: Aleksandra D. Radina et al, Theoretical study of adsorption properties and CO oxidation reaction on surfaces of higher tungsten boride, Scientific Reports (2024). DOI: 10.1038/s41598-024-63676-7
Journal information: Scientific Reports
Provided by Skolkovo Institute of Science and Technology