This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Autophagic organelles restrict mouth size to regulate cellular clean-out, study reveals
Autophagy, which literally means "self-eating," is a cellular cleaning-out process that maintains our bodies in good order, but excessive autophagy can be too much of a good thing.
Now Weizmann Institute of Science researchers have revealed a dietary control device—one that keeps the mouth of the autophagic machinery from opening too much, preventing it from eating everything in sight. The study is published in Developmental Cell.
"The mechanism we discovered enables autophagy to devour as much damaged material as needed, but not more," says Prof. Zvulun Elazar of Weizmann's Biomolecular Sciences Department, who headed the research team.
Autophagy's specialty is the removal of large structures, such as damaged protein aggregates or fractions of organelles. In extreme situations such as starvation, it can degrade bits and pieces of random cellular material to provide the cell with essential building blocks for proper maintenance of its ongoing vital processes.
Because autophagy is essential to so many body systems, particularly to the maintenance of long-lived cells that no longer divide, such as neurons, faults in this cellular housekeeping can lead to a variety of diseases. Defects in autophagy are known to contribute, for example, to the death of neurons in Parkinson's and other neurodegenerative diseases.
The decline in autophagy that occurs with age is also thought to increase the risk of disease, including cancer. A better understanding of the mechanisms that regulate autophagy may help develop new therapies, but simply enhancing autophagy might not always be the best solution.
"In cancer, for example, autophagy is a double-edged sword," Elazar explains. "Insufficient autophagy leads to the build-up of free radicals, which contribute to cancer, but then cancerous tumors rely on autophagy to survive."
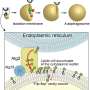
The new study in Elazar's lab, led by Ph.D. student Oren Shatz, sought to determine how the body regulates autophagy. Since the needs of the cells change constantly, the autophagic machinery—an organelle called the autophagosome—is built each time from scratch and broken down once its job is done.
The membrane of the autophagosome precursor structure, called the phagophore, engulfs the material to be removed and hauls it over to a "trash dump," the lysosome, where it is degraded.
Some phagophores swallow whatever gets in their way in a nonselective manner. For others, eating is highly selective, in which case they are aided by proteins that spoon-feed them, guiding specific structures into their mouths.
Until now, the accepted view was that in both cases, the phagophore's mouth opens to the max to gobble up whatever needs to be eaten. The findings of the Weizmann study, conducted on yeast, suggest that this view does not come anywhere near the true eating habits of the average autophagosome.
"We've discovered a mechanism that controls the opening of the phagophore's mouth," Shatz says. "This is especially crucial in the case of nonselective autophagy, which is potentially dangerous because it could mistakenly eat the entire cell from within."
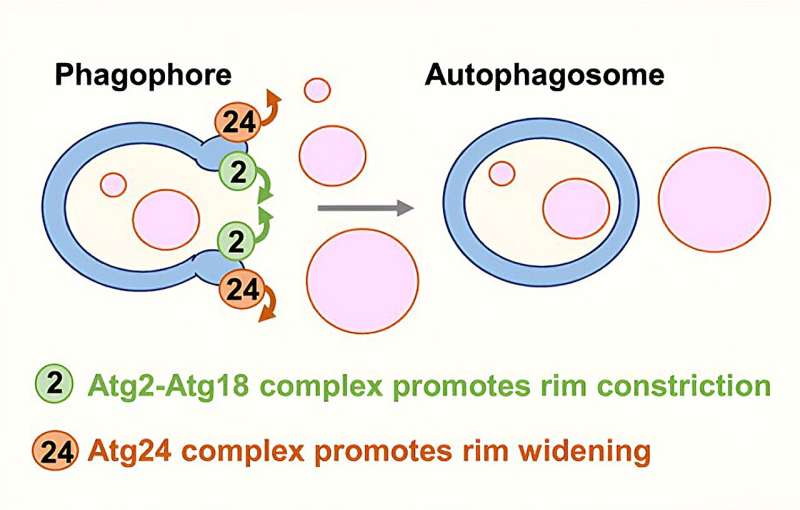
The newly revealed mechanism ensures that in nonselective autophagy, the autophagosome can't open gaping jaws to swallow everything in its path. Rather, as the membrane begins to be built, the size of its opening is limited—thereby restricting what can be swallowed—even as the membrane itself continues to grow, expanding until enough material for removal is captured.
As a result, contrary to the classical view, phagophores are not shaped like a tea cup—that is, with a mouth that takes up their entire circumference—but more like an amphora, a shape most familiar from ancient Greek vases, with a narrow, neck-like rim.
No matter how big the "vase" is, the opening remains permanently narrow until the autophagosome has performed its duty and is once again broken down. In other words, rather than a gluttonous eater, the autophagosome is a delicate diner, and the "food" enters by diffusion.
The researchers further showed that even in selective autophagy, in which extra caution is secured by the proteins that pick out the hazardous material, the phagophore does not open its mouth uncontrollably. The size of the opening is regulated by the same mechanism, even though it does end up wider than in the nonselective process.
Ultimately, in both types of autophagy, selective or not, once the organelle has eaten its fill, this mechanism is dismantled so that the opening can shut down, sealing the membrane and allowing the swallowed material to be digested inside.
Shatz and colleagues then revealed the molecular details of this control mechanism. It involves two major protein complexes: one called Atg24-Atg20, which makes the opening larger, and another, Atg2-Atg18, which makes it tighter.
The activity of both complexes was found to be coordinated through their interaction with PI3P, a well-established autophagic signaling chemical: The two complexes compete for binding to the PI3P molecules, which serve as adhesive pads.
Having identified these major players, the scientists showed that they could manipulate them in order to enlarge or shrink the size of the phagophore opening on demand.
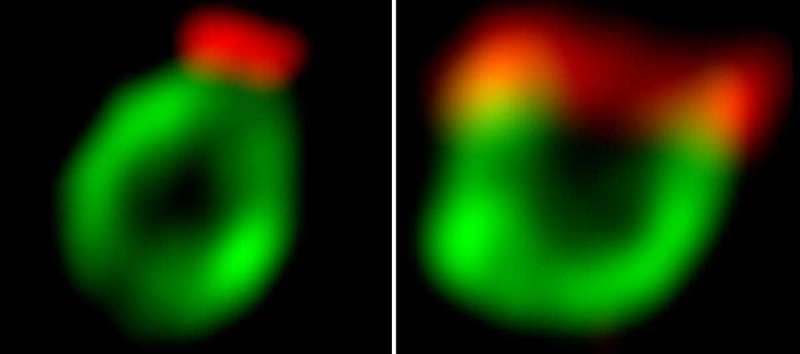
These revelations were made possible by a number of sophisticated advances achieved in the course of the study. In particular, Shatz developed ways of augmenting the activity of the Atg24-Atg20 complex and reducing the activity of the Atg2-Atg18 complex, rather than overexpressing or completely knocking out their genes, as is commonly done in molecular cell biology.
In addition, he and his colleagues developed an innovative way of labeling several proteins with different colors within the same experiment.
The study's findings contribute to an in-depth understanding of autophagy that in the future might open the way to medical applications. "If you want to fix a car, you need to know its parts and exactly what they do," Elazar says. "Similarly, we need to arrive at a detailed knowledge of autophagy mechanisms to be able one day to tweak them to just the right levels in our bodies."
More information: Oren Shatz et al, Rim aperture of yeast autophagic membranes balances cargo inclusion with vesicle maturation, Developmental Cell (2024). DOI: 10.1016/j.devcel.2024.02.002
Journal information: Developmental Cell
Provided by Weizmann Institute of Science