This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New model finds previous cell division calculations ignore drivers at the molecular scale
When a single bacterial cell divides into two during periods of rapid growth, it doesn't split in half once it reaches a predetermined size. Instead, data has shown, a cell will divide once it has added a certain amount of mass.
The two processes sound similar, but they each carry different risks. Many researchers believed it was a safer bet for the cell if it split once it reached a certain size.
New mathematical modeling from the Kenneth P. Dietrich School of Arts and Sciences shows the risks may have been miscalculated, however, because previous calculations have ignored the drivers of cell division at the molecular scale. Their findings were published in the journal Physical Review Letters.
"Trying to hit a target size before dividing seems like the best strategy for maintaining a precise cell size," said Andrew Mugler, associate professor in the Department of Physics and Astronomy. "But when you look at what bacteria do, it looks like they use the second-best strategy."
Dividing each time a cell reaches a target size, known as the sizer strategy, seemed to be the best way to constrain the size of a cell. If something goes wrong and the cell gets too big or too small, it could be easily sorted out in the next generation—it just needs to get back to its target size.
But if something goes wrong using the so-called adder strategy, the cell takes longer to get back to its original size. That's because the method relies on knowing how much a cell grew since its last division. Overall, the adder was thought to result in a less precise size distribution.
So why do cells use this adder method?
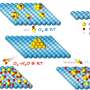
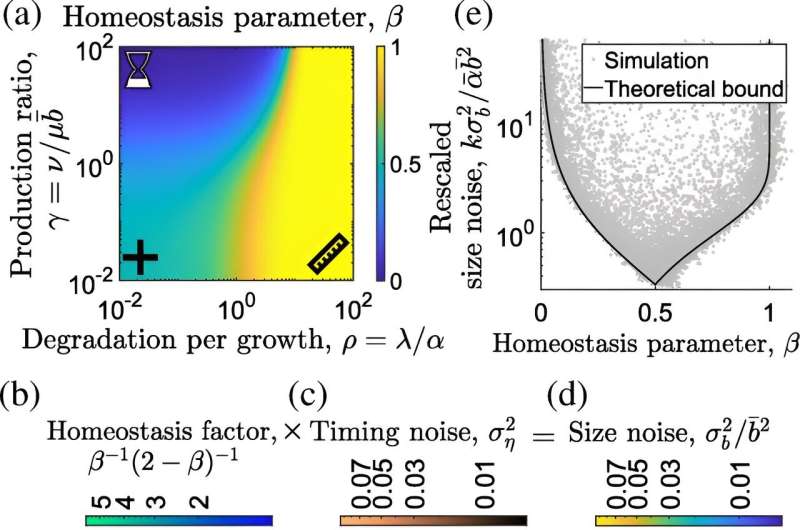
First author Motasem ElGamel, a doctoral student in the Department of Physics and Astronomy, designed a model that goes beyond the scale of the cell and includes molecular level changes as well. He found the adder method is more precise, researchers have just misunderstood its true nature.
Cells don't divide when they grow a certain amount of mass, but when they add a specific number of a certain molecule. The two measures do grow in parallel—more molecules equals more mass—but considering them together has turned out to be key.
When ElGamel took the number of molecules into account alongside the additional mass, the adder strategy was more precise and less sensitive to mistakes during replication.
"Until this point, models primarily accounted for variables at the scale of the whole cell—its size, or the time it took to divide, etc. But we know the cell makes these decisions based on amounts of certain molecules in the cell body," Associate Professor Mugler said. "That's what needed to be incorporated."
More information: Motasem ElGamel et al, Effects of Molecular Noise on Cell Size Control, Physical Review Letters (2024). DOI: 10.1103/PhysRevLett.132.098403
Journal information: Physical Review Letters
Provided by University of Pittsburgh