This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Operando spectroscopy provides a window on water oxidation
Iridium oxide catalysts are effective for water oxidation making them very attractive for green technologies. A team including researchers from SANKEN (The Institute of Scientific and Industrial Research) at Osaka University has therefore taken the closest look yet at how they work.
In a study published in the Journal of the American Chemical Society the team used spectroscopy to reveal how the chemical species involved in iridium oxide-catalyzed oxygen evolution reaction (OER) interact with the solution around them.
The OER is important in many clean energy processes such as turning carbon dioxide into usable liquid fuels and generating green hydrogen from the electrolysis of water. Both processes will be crucial in a future without fossil fuels. Therefore, thoroughly understanding the OER is an important research focus.
Catalytic processes can be complex with various intermediate species involved in getting from the starting material to the desired product. Operando techniques allow these intermediates to be investigated using spectroscopy during the reaction, providing a window into what is actually happening.
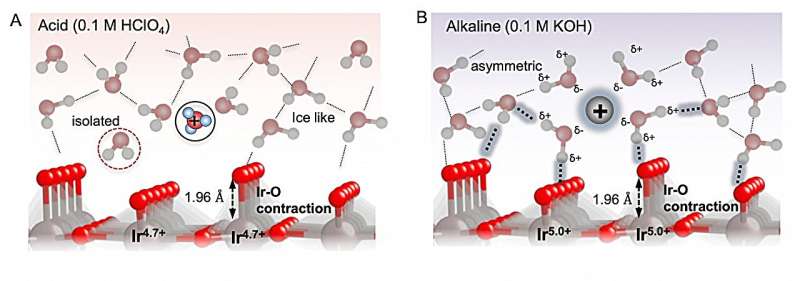
Using an electrode with an iridium oxide surface, the researchers investigated the oxidation of water molecules in solutions with different pH values.
"Interaction between the electrode surface and the oxygenated intermediates is key to the efficiency of the OER, so optimizing the catalyst material has generally been the focus," explains senior author Reshma R. Rao from Imperial College London.
"However, the observations to date have left unanswered questions, so we have taken a closer look at the solution side of the interface using operando UV-Vis spectroscopy, X-ray absorption spectroscopy, and surface-enhanced infrared spectroscopy."
To achieve efficient reaction, binding of reaction intermediates to the electrode must be just right to allow the intermediates to interact with the electrode, but not be so strong that they get stuck to the electrode and can't react. The researchers found that the binding was controlled by long-range interactions between the intermediates through the solution and this depended on the pH.
In alkaline conditions, water near the electrode influenced the long-range interactions between the oxygenated species, which affected their binding to the surface. So, although the intermediates bound more strongly at higher pH, the interactions facilitated by interfacial water destabilizes the oxygenated species and allows the reaction to take place.
"Using operando spectroscopy and complementary techniques to get a direct look at the species involved has allowed us to broaden the understanding of catalyst performance beyond electrode binding," says senior author Yu Katayama. "We believe that such insight will be the key to optimizing the kinetics of the OER."
The findings will contribute to increasing the efficiency of water oxidation for green hydrogen production. In addition, combining operando spectroscopy with complementary techniques may be useful for understanding the catalysis of many other processes.
More information: Caiwu Liang et al, Role of Electrolyte pH on Water Oxidation for Iridium Oxides, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c12011
Journal information: Journal of the American Chemical Society
Provided by Osaka University