This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Constructing 'on-gel' alveolar organoids as a new screening platform
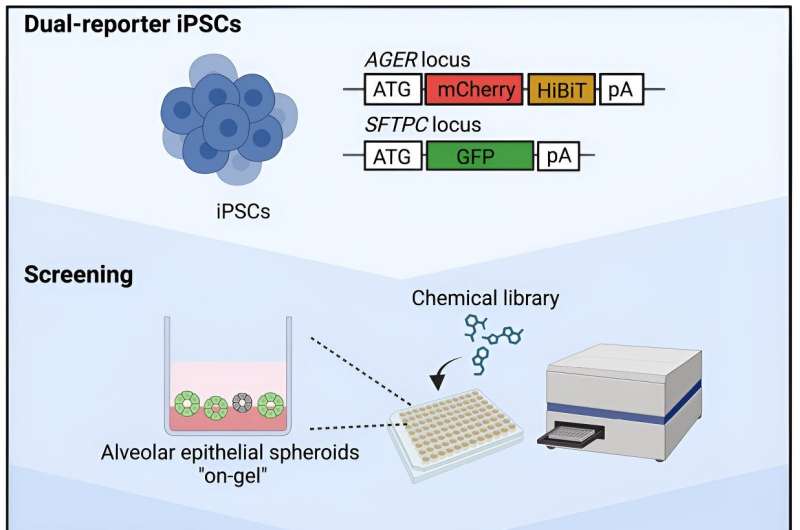
A study led by Professor Shimpei Gotoh (Department of Clinical Application), introduces a new culturing method to generate alveolar organoids suitable for medium- and high-throughput screening and identified several chemicals with synergistic effects on AT1 cell differentiation. The work is published in the journal Stem Cell Reports.
Alveoli, the functional units of the lung where gas exchange occurs, are primarily composed of alveolar type 1 and 2 epithelial cells (AT1 and AT2 cells, respectively). In addition to producing pulmonary surfactants, AT2 cells are cuboidal tissue stem cells that maintain alveolar homeostasis and regulate regenerative processes following injury by self-renewal and differentiation into AT1 cells.
In contrast, AT1 cells are thin and flat cells that form most of the alveolar surface to mediate the exchange of oxygen and carbon dioxide. Current knowledge of the signaling mechanisms underlying AT1 differentiation from AT2 cells is based primarily on mouse studies, and it remains unclear whether there are species differences in the regulatory process.
Furthermore, although studies have traditionally employed human primary AT2 cells, their limited supply has impeded progress in this research area. Alternatively, human iPS cells represent a valuable starting material for studying alveolar epithelial cell differentiation.
The Gotoh Laboratory had previously established induction protocols to generate AT2 cells from iPS cells and create alveolar organoids from patient-derived iPS cells for disease modeling. However, such organoids are not optimal for screening procedures because they require large cell numbers and are totally embedded in Matrigel, a cell culture substrate resembling the extracellular environment.
In the present study, the researchers generated a novel dual reporter iPS cell line monitor AT1 cell differentiation and derived a new "on-gel" method to culture alveolar organoids by seeding iPS cell-derived lung progenitor or AT2 cells on top of Matrigel in 96-well plates.
While other AT2 markers showed similar expression using this method, AT2 cells in the resulting spheroids notably expressed higher levels of surfactant protein C than alveolar organoids generated using previously established protocols. Conversely, multiple AT1 cell markers were detected at lower levels in the "on-gel" spheroids, suggesting their absence or immaturity.
Although cells positive for AT1 cell markers were detected, transcriptomic analyses confirmed a more AT2-dominant composition for these "on-gel" spheroids. Furthermore, spheroid AT2 cells exhibited structural characteristics reminiscent of functional AT2 cells.
Altogether, these observations suggested that the new "on-gel" culture method, requiring a small number of cells to generate and easier handling, is optimal for screening procedures to identify potential inducers of AT1 differentiation.
As a proof-of-principle, the research team performed a medium-throughput chemical screen with 274 compounds using lung progenitor cells grown using their newly developed "on-gel" method and found 15 chemicals to have significantly increased the levels of the AT1 cell-specific reporter.
They validated these candidate compounds next by examining their ability to enhance the expression of AT1 cell-specific genes, AGER, CLIC5, and CAV1.
Among them, LATS-IN-1 and ROCK-IN-2, known as inhibitors of LATS1/2 and ROCK2, respectively, significantly elevated these AT1 marker genes. In addition to the finding that LATS1/2 inhibition promoted AT1 differentiation by activating YAP/TAZ signaling, the researchers also found ROCK-IN-2 to induce YAP/TAZ-related gene expression. However, both candidate compounds clumped the spheroids and promoted proliferation, properties opposite of AT1 cells (i.e., flat and thin quiescent cells).
They also tested other ROCK2 inhibitors, which did not promote AT1 differentiation, suggesting the ability of ROCK-IN-2 to induce AT1 differentiation is likely attributable to an off-target effect on the YAP/TAZ pathway.
Four other candidate compounds demonstrated intermediate effects on AT1 marker gene expression and were thus tested for synergistic effects in combination with LATS-IN-1 to activate YAP/TAZ signaling. Notably, BAY1125976, an allosteric AKT inhibitor, synergistically enhanced AT1 gene expression when combined with LATS-IN-1.
During validation, the researchers also observed synergistic effects between LATS-IN-1 and two other PI3K/AKT inhibitors, Alpelisib and AZD6482.
Similar findings were observed when an independent human iPS cell line or primary AT2 cells were treated with the same chemical combination. Through additional transcriptomic, morphological, and functional analyses, the researchers consistently found the co-treatment of LATS-IN-1 and BAY1125976 to promote cellular changes toward an AT1 cell-like phenotype.
By generating a new dual reporter iPS cell line to monitor AT1 differentiation and establishing the novel "on-gel" spheroid culture method compatible with high-throughput screening, this recent work has identified chemical modulators of YAP/TAZ and AKT signaling pathways as a potent inducer mix for AT1 cell differentiation.
With the possibility to adapt "on-gel" spheroid cultures for high-throughput chemical or CRISPR/Cas-based genetic screens, the innovations developed in this study will help propel the next breakthroughs in regenerative pulmonary medicine.
More information: Yuko Ohnishi et al, Screening of factors inducing alveolar type 1 epithelial cells using human pluripotent stem cells, Stem Cell Reports (2024). DOI: 10.1016/j.stemcr.2024.02.009
Journal information: Stem Cell Reports
Provided by Kyoto University