This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Exploring the transferability of extracytoplasmic function switches across bacterial species
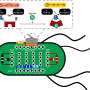
![(A) The ECF profile of S. meliloti is compared to the heterologous ECF switches that were shown to be active in the Rm1021 wild type and/or the ECF/anti-σ-free strain. Phylogenetic groups are given on top. If 2 groups are listed, the first one refers to the initial classification [13] and the second one to the latest classification [12]. Numbers indicate the presence of one or more ecf genes from a certain phylogenetic group in the genome of S. meliloti. * is indicative of an active heterologous ECF switch. Red shaded boxes label shared phylogenetic ECF groups. (B) Performance of the ECF26-based switch in Rm1021 and the ECF/anti-σ-free strain and (C) Pecf26 activation by endogenous ECFs from the same phylogenetic group (RpoE1, RpoE3, RpoE4, and RpoE6) assayed in the ECF/anti-σ-free strain. The experimental setup in (B) and (C) corresponds to the setup described in Fig. 1A. IPTG at different concentrations (0, 50, and 500 μM) was used to induce ecf expression. Promoter activities were normalized to yield luminescence units per unit of OD600 (left y-axis). Each dot represents the mean response of 3 biological replicates 6 h after the addition of IPTG. Arrow bars show the standard deviation. Rectangular bars represent the average log2(fc) of promoter activity in the presence of 500 μM IPTG compared to the basal promoter activity in the absence of any heterologous ecf (right y-axis). The underlying data are given in Table S9. Credit: BioDesign Research (2023). DOI: 10.34133/bdr.0025 Exploring the transferability of extracytoplasmic function switches across bacterial species](https://scx1.b-cdn.net/csz/news/800a/2024/exploring-the-transfer.jpg)
Extracytoplasmic function sigma factors (ECFs) have been successfully used for constructing predictable artificial gene circuits in bacteria like Escherichia coli, but their transferability between species within the same phylum remained unknown.
Now, a recent study by a group of researchers from Germany and Australia explored the bacteria Sinorhizobium meliloti and identified ECF switches with cross-species functionality, constructed genetic circuits, and provided a toolbox for universal synthetic biology applications.
In the field of synthetic biology, creating artificial gene circuits with predictable outcomes is both a challenge and a necessity. Extracytoplasmic function sigma factors (ECFs) have garnered significant attention for their pivotal role in initiating transcription in bacteria, especially under stress conditions. Extensive research has categorized different groups of ECFs, showcasing their potential to construct multi-step genetic circuits with delayed gene activation.
While these circuits have shown success in well-studied bacteria like Escherichia coli, the degree to which ECFs can be transferred across species within the same phylum has remained uncertain.
To address this gap, Professor Anke Becker from the Center for Synthetic Microbiology (SYNMIKRO) and the Department of Biology, Philipps-Universität, Germany, and her team investigated the transfer of ECF switches from E. coli to the α-proteobacterium Sinorhizobium meliloti. Their study was published in BioDesign Research.
The team tested 20 different ECF switches in S. meliloti bacteria, which had previously demonstrated functionality in E. coli. The switches were named based on their origin and had systematic identifiers. They introduced these switches into two types of S. meliloti strains—one was the normal wild type, and the other was a modified strain without its own ECF switches.
They found that ECF switches from E. coli could be successfully transferred to S. meliloti with a success rate of over 50%. Importantly, these switches retained their functionality and pattern of orthogonality in both host species. Factors such as transcription rates, translation, protein stability, and host-specific characteristics were found to influence the functionality of ECF switches in S. meliloti.
"We were pleased to observe such high transferability and functionality of the ECF switches across species. This suggests that synthetic biology approaches developed in one bacterial species can potentially be applied to a wide range of organisms, expanding the scope of genetic engineering," explains Prof. Becker.
The study underscores the importance of understanding both the genetic elements and the host environment when engineering synthetic biological systems. By comprehensively investigating these factors, researchers can enhance the predictability and reliability of synthetic biology applications.
Another key finding of the study was the broad phylogenetic acceptance range of ECF switches observed in S. meliloti and E. coli. Unlike some bacterial species, which exhibit narrow acceptance ranges for heterologous ECF switches, S. meliloti and E. coli displayed a remarkable tolerance to switches from diverse bacterial classes and species.
This suggests that these species could serve as universal hosts for synthetic biology applications, potentially facilitating the development of novel biotechnological solutions.
In addition to experimental validation, the researchers employed computational predictions to preselect suitable ECF/promoter pairs for transfer between bacterial hosts. These predictions, combined with experimental data, provided valuable insights into the design of genetic circuits with minimal crosstalk and optimal performance.
By leveraging computational predictions alongside experimental validation, researchers can accelerate the design-build-test cycle and streamline the development of complex genetic circuits.
The study also introduced a set of single-copy plasmid vectors for modular assembly of genetic circuits in S. meliloti. These vectors, compatible with the Molecular Cloning (MoClo) DNA assembly method—a modular cloning method used in synthetic biology for the precise and efficient assembly of DNA fragments into larger constructs, offer a standardized platform for genetic engineering in this bacterial species.
"Our MoClo-compatible plasmid vectors provide researchers with a versatile toolkit for constructing genetic circuits in S. meliloti. These vectors streamline the assembly process and facilitate the iterative optimization of genetic circuits, ultimately accelerating the pace of synthetic biology research," states Prof. Becker.
Overall, the study represents a significant step forward in the field of synthetic biology, demonstrating the transferability of genetic switches across bacterial species and providing valuable insights into the design and engineering of complex genetic circuits. With further research and development, these findings hold the potential to revolutionize various industries and address pressing challenges in biotechnology and beyond.
More information: Doreen Meier et al, A MoClo-Compatible Toolbox of ECF Sigma Factor-Based Regulatory Switches for Proteobacterial Chassis, BioDesign Research (2023). DOI: 10.34133/bdr.0025
Provided by NanJing Agricultural University