This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Unlocking the secrets of synthetic biology: Host physiology over phylogeny in genetic circuit performance
Broad host range (BHR) synthetic biology aims to capitalize on a wide range of microbial phenotypes to expand biodesign applications not confined to traditional model organisms. Despite the ever-expanding genetic toolkit, reliance on a small number of model hosts has limited innovation, as highlighted by the "chassis effect," where identical genetic circuits perform differently in various organisms.
Current research exploring novel microbial hosts highlights this variability, posing challenges to predictability and circuit optimization. Addressing the knowledge gap in biological determinants driving the chassis effect is crucial for enhancing predictability and expanding Biodesign applications in BHR synthetic biology.
In August 2023, BioDesign Research published a research article titled "Revealing the Host-Dependent Nature of an Engineered Genetic Inverter in Concordance with Physiology."
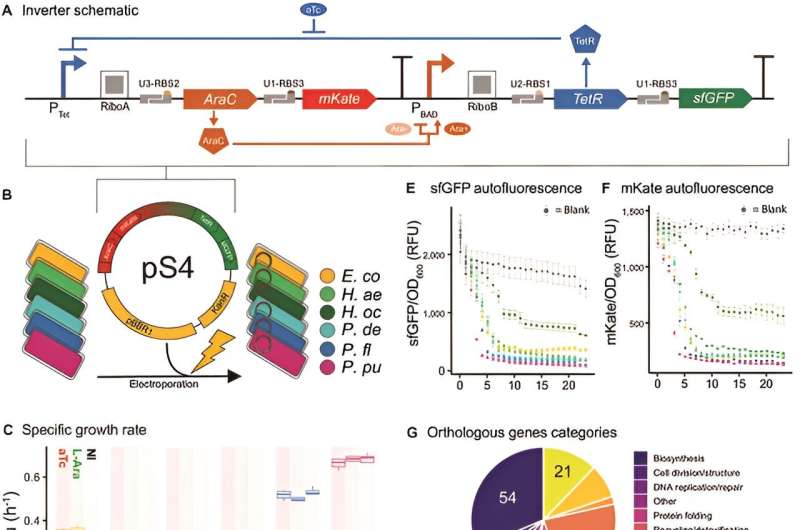
In this study, researchers sought to understand whether phylogenomic relatedness or host physiology can more accurately predict the performance of genetic circuits in bacterial hosts. The study revealed distinct differences in growth physiologies and molecular characteristics across the species, significantly impacting the inverter's performance.
It was found that hosts with similar growth and molecular physiology metrics showed comparable inverter performances, thus linking bacterial physiology directly to the chassis effect. This discovery is pivotal for broad-host-range synthetic biology, enhancing the predictive power for implementing genetic devices in novel microbial hosts. The study's core involved a detailed comparative analysis of host physiology and phylogeny.
Various physiological parameters like growth rate, gene copy number, and codon usage bias were quantified and correlated with the inverter circuit's performance in each host. Contrary to conventional focus on phylogenomic relatedness, the research demonstrated that host physiology is a more significant predictor for circuit performance.
This was further substantiated by multivariate statistical techniques, including the Mantel test and Procrustes Superimposition analysis, which conclusively pointed out the predominance of physiological factors over phylogenomic relatedness in determining the performance of genetic devices.
In conclusion, this study challenges the traditional reliance on phylogeny for predicting microbial phenotypes, underscoring the critical role of physiological factors. The findings in this work are instrumental in synthetic biology, suggesting a paradigm shift in how the performance of genetic devices can be predicted and optimized in diverse microbial hosts.
By highlighting the significance of host-specific physiological traits, this research shows a new direction for more accurate and effective implementation of genetic devices. This novel approach not only enhances the understanding of the chassis effect but also marks a significant stride in broadening the utility of synthetic biology across a wider range of bacterial hosts.
More information: Dennis Tin Chat Chan et al, Revealing the Host-Dependent Nature of an Engineered Genetic Inverter in Concordance with Physiology, BioDesign Research (2023). DOI: 10.34133/bdr.0016
Provided by NanJing Agricultural University