Generating antifungals via bacterial conjugation to yeast
The ever-diverse fungi play several key roles in our day-to-day life. From facilitating ecological nutrient cycling, to being used in industrial manufacturing and being a key ingredient in our food, fungi wear many different hats. Among all fungal species, yeasts are the best-studied and easiest to domesticate by humans.
For example, the yeast species Saccharomyces cerevisiae (S. cerevisiae) is not only used as the primary fermenter for beer, bread and wine, but is also a critical component in the production of insulin, vaccines, and essential recombinant proteins. But not all yeast and fungi are human-friendly and tamable. Several species are opportunistic disease-causing pathogens, associated with mild–to–severe fungal infections and even cancer.
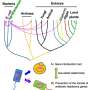
Plasmids are small genetic structures that are independent from the chromosomes and can replicate on their own. They can be used to introduce synthetic, modified genes into the cells of other organisms. Plasmid delivery through conjugation between bacterial species has proven effective in creating novel antimicrobial agents, targeting specific genes to eliminate or suppress pathogens.
However, this approach has eluded scientists when it comes to dealing with yeasts, which are less controllable. Therefore, it has become imperative to study and manipulate the biology of fungal species for critical biotechnological applications, especially the development of antifungal drugs.
In response to this unmet requirement, a team of researchers from Canada have developed and optimized the transfer of superior conjugative plasmids between bacteria and different yeast species via conjugation.
"To create our novel plasmids, we built derivatives of the conjugative plasmid, pTA-Mob 2.0, using designed gene deletions and cluster mutations to improve bacterial conjugation with yeasts," explained Dr. Bogumil J. Karas, Assistant Professor at University of Western Ontario and corresponding author of the study that was published in BioDesign Research.
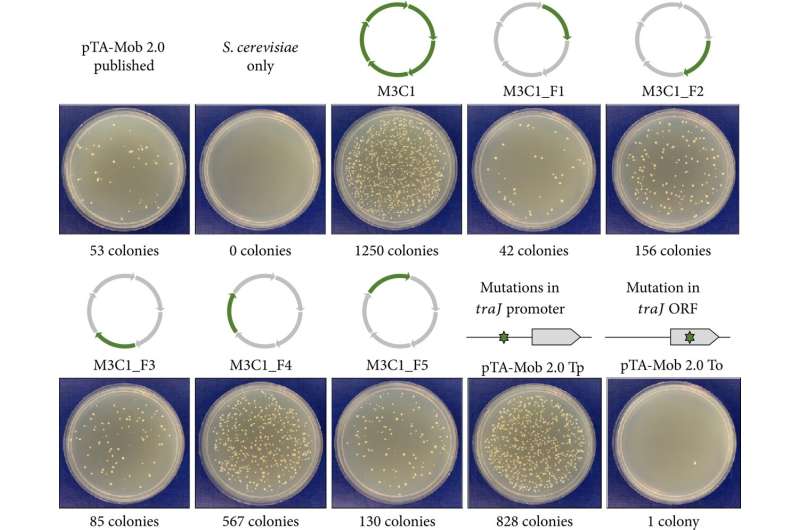
pTA-Mob 2.0 is composed of genetic elements required for plasmid maintenance and transfer, making it ideal for this study. The team first optimized this plasmid for bacteria–to–yeast conjugation by deleting 55 genes or small genetic regions to create four streamlined plasmids: M1–M4, with two clones each. These modified plasmids were then transferred from Escherichia coli (E. coli) bacteria to S. cerevisiae via conjugation and assessed based on the yeast colony formation.
Plasmid M3 clone 1 (M3C1) showed the most significant increase in conjugation efficiency. The mutations that contributed to this increased efficiency were found to be in the promoter region of the conjugative gene traJ. This mutation lowered the expression of traJ, which significantly impacted the expression of other conjugative proteins, thereby promoting conjugation.
Thereafter, five derivative plasmids of M3C1 were created containing the traJ mutation, including the pSuperCon5 (pSC5) plasmid with additional elements for improved conjugative transfer to diverse yeast species and diatoms. pSC5 also had antibiotic selection markers added to it.
As compared to the original pTA-Mob 2.0 plasmid, the bacteria-yeast conjugation frequency for pSC5 was 10- and 23-fold more when tested in cis (which mobilizes itself) and trans (which mobilizes another plasmid) setups, respectively. This could be because the bacteria had fewer adverse effects on the yeast when it was carrying the pSC5 plasmid.
This enhanced conjugation was further replicated with a different bacterial species—Sinorhizobium meliloti, as the pSC5-carrying donor, suggesting that this mechanism can be used with different bacteria. Furthermore, the pSC5 plasmid allowed for successful DNA transfer to seven yeast species, including Candida auris—a known pathogen—albeit with varying levels of efficiency.
Subsequently, the pSC5 plasmid was domesticated for Golden Gate Assembly—a molecular cloning method enabling the simultaneous and directional assembly of multiple desirable DNA fragments into a single piece. Following this, the researchers verified and proved that their novel bacteria–to–yeast conjugation method and improved conjugative plasmids can be used to deliver antifungal therapies with high efficiency.
The team is now looking at integrating their trans-kingdom conjugation system with CRISPR-based genetic editing platforms to target pathogenic fungi in different environmental niches.
Talking about the success and future applications of this research, Dr. Karas said, "Conjugation-based techniques such as the one we developed provide a unique and functional method to deliver plasmids between microbial species in vitro and in vivo. With additional enhancements in the conjugation frequency, our designer conjugative plasmids could be used as antifungal reagents, with important applications for the development of next-generation antimicrobial drugs."
More information: Ryan R. Cochrane et al, Superior Conjugative Plasmids Delivered by Bacteria to Diverse Fungi, BioDesign Research (2022). DOI: 10.34133/2022/9802168
Provided by BioDesign Research