This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Intensifying the production of high-value compounds from industrial waste
A study by the Center for Integrated Technology and Organic Synthesis (CiTOS) demonstrates how glycerol carbonate, a biosourced industrial additive, can be produced in record time using CO2 and a by-product of the cooking oil recycling industry.
This study, conducted in collaboration with a team from the Center for Studies and Research on Macromolecules (CERM) under the umbrella of a Concerted Research Action and published in Angewandte Chemie International Edition, lays the foundations for continuous industrial production.
Ambitious R&D and production directives in Europe are stimulating the integration of innovative technologies to reduce environmental impact and to move away from an exclusive reliance on petrochemical resources. In this context, researchers at CiTOS, led by Jean-Christophe Monbaliu, are developing new processes that privilege molecules derived from biomass.
Glycerol is a prime target among these biobased molecules due to its abundance. Glycerol is mainly derived from the biodiesel industry and cooking oil recycling; its low economic value has relegated it to the status of waste until now. Another waste turned public enemy number one, CO2, is an industrial gaseous effluent with low economic value.
By combining their respective areas of expertise, the teams at CiTOS (continuous flow organic chemistry in micro/mesofluidic reactors and upgrading of biobased compounds) and CERM (synthesis of organic materials from CO2) are developing new methods to valorize glycerol and CO2 toward high value-added molecules.
Glycerol carbonate, which formally results from the condensation glycerol and CO2, has recently become a rising star. It offers several advantages over other petroleum-based carbonates such as ethylene and propylene carbonates, which are key electrolyte carriers in lithium batteries.
Its significantly lower flammability could greatly reduce the fire risks inherent in these batteries. The carbonate can also be used as a biolubricant, formulation agent, or alternative green solvent. "Despite such potential, the current market for glycerol carbonate remains very limited," comments Jean-Christophe Monbaliu. "The main reason is that current production processes are slow and expensive. Our work is in the process of changing that."
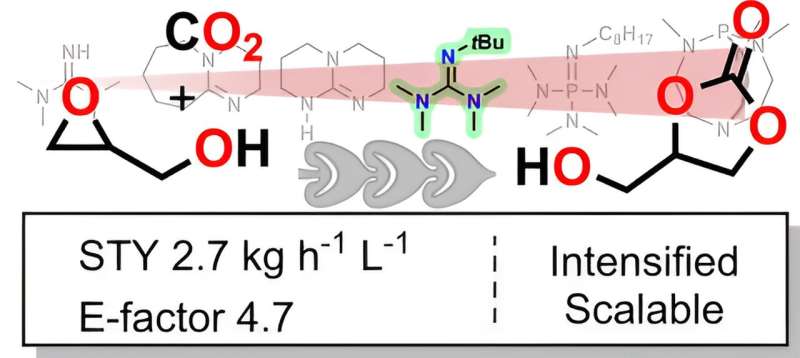
The work is based on a hybrid approach combining fundamental and applied organic chemistry: A detailed study of the mechanism through quantum chemistry and its deployment under mesofluidic conditions converge toward a unique intensified process. The process, validated at the pilot scale, transforms a direct derivative of glycerol, namely glycidol, in the presence of CO2 and an organic catalyst into glycerol carbonate.
The efficiency of the process, which reaches completion in less than 30 seconds, far surpasses all current processes for glycerol carbonate production. "Such favorable metrics open unprecedented perspectives for potential future industrialization," concludes Jean-Christophe Monbaliu.
More information: Claire Muzyka et al, Intensified Continuous Flow Process for the Scalable Production of Bio‐Based Glycerol Carbonate, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202319060
Journal information: Angewandte Chemie International Edition
Provided by University de Liege