This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Acetalization: A feasible and sustainable strategy for biomass valorization
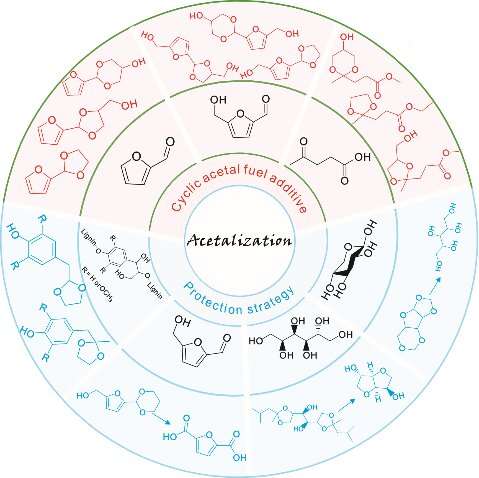
Biomass, mainly composed of lignocellulose and vegetable oil, has been acclaimed as one of the most promising sustainable sources of raw carbon material for the synthesis of transport fuels and value-added chemicals. The catalytic conversion of lignocellulose/vegetable oil and their related derivatives has attracted great attention in biomass valorization.
Many elegant methods including hydrolysis, dehydration, hydrogenation, hydrogenolysis, oxidation, etherification, esterification, amination, aldol condensation, Diels–Alder, Knoevenagel condensation, and acetalization have been developed for the valorization of lignocellulose/vegetable oil derivatives toward value-added chemicals and biofuels.
In particular, acetalization is advocated as an appealing approach in biomass valorization because it serves as both a synthesis tool for renewable acetal fuel additives and a protection strategy to improve product selectivity. A team of scientists summarized the latest progresses about the application of acetalization strategy in biomass valorization. Their work is published in Industrial Chemistry & Materials.
"The development of efficient and selective strategies is crucial for valorizing lignocellulose/vegetable oil derivatives," said Changzhi Li, a Professor at Dalian Institute of Chemical Physics, Chinese Academy of Sciences, "In this review, we systematically discussed the recent advancement of the application of acetalization strategy in biomass valorization."
"The latest progresses in the development of catalytic systems for the acetalization of biobased furanic compounds and biogenic ethylene glycol/glycerol are systematically summarized and discussed, with an emphasis on the reaction pathway, relationship between catalyst structures and their performance, and relevant catalytic mechanism."
"Moreover, the application of the acetalization strategy for protecting carbonyl groups/diol structure functionalities to improve the target products' selectivity in lignin depolymerization, 5-hydroxymethylfurfural oxidation, sorbitol dehydration, and xylose hydrogenation is highlighted. We also provided an outlook on the remaining challenges to this field."
"Acetalization, a well-known reversible reaction between carbonyl compounds and alcohols, usually need excess of one of the reactant to compel reversible acetalization completion," Li said.
"Nevertheless, studies on the recovery of excess reactant after the reaction are scarce. Moreover, the separation and purification of cyclic acetals/ketals deserve much attention. Expectedly, rectification or designing a suitable biphasic reaction system for this transformation may probably realize the recovery of the surplus substrate and/or separation of the acetals product."
"A five-membered-ring acetal (i.e., 1,3-dioxolane) and a six-membered-ring acetal (i.e., 1,3-dioxane) are available from the acetalization of furanic compounds and glycerol," Li said, "However, it is still a significant challenge to achieve the selective synthesis of 1,3-dioxolane or 1,3-dioxane. Designing structurally adjustable catalysts or choosing suitable solvents may provide an opportunity for achieving the selective synthesis of 1,3-dioxolane or 1,3-dioxane."
"In addition, the use of crude glycerol, stemming from biodiesel production, for acetalization is more economically viable, and the influence of impurities on the acetalization reaction needs to be investigated."
At present, the available researches for getting insights into detailed catalytic mechanisms for the acetalization of furanic compounds and ethylene glycol/glycerol are limited. More effort should therefore be devoted to the fundamental understanding of the catalytic mechanism via in situ spectroscopic measurements and density functional theory calculation.
"The synthesis of a single product with high selectivity during the valorization of biomass derivatives is very challenging due to the presence of multiple functional groups (e.g., C=O, C=C, and C-O) in biomass molecules," Li said.
"Taking advantages of the fact that acetalization is a reversible reaction and the formed cyclic acetals/ketals are stable/low reactivity in basic media, acetalization as a protection strategy of the carbonyl group is worth further promotion in biomass valorization such as exclusive hydrogenation of C=C in biobased multifunctional compounds while leaving the C=O group unreduced."
"In this review, our main goal is to provide readers with timely and accurate research progress on the application of acetalization strategy in biomass valorization," Li said.
More information: Jian He et al, Acetalization strategy in biomass valorization: a review, Industrial Chemistry & Materials (2023). DOI: 10.1039/D3IM00050H
Provided by Industrial Chemistry & Materials