Unlocking the organic chemistry of anhydrous dinitrogen trioxide through continuous flow process technology
Researchers at CiTOS—Center for Integrated Technology and Organic Synthesis (University of Liège, BE) led by Jean-Christophe Monbaliu have devised an on-demand flow platform for the generation of anhydrous dinitrogen trioxide (N2O3), a very potent nitrosation reagent notoriously challenging to prepare and to use.
This project unlocks the potential of N2O3 for the preparation of high value-added organic molecules. The results of this study are now published in the journal Angewandte Chemie International Edition as a research paper.
Small cyclic molecules containing nitrogen atoms (N-heterocycles) are common structures in bioactive compounds, especially in active pharmaceutical ingredients. Developing new methods for the incorporation of nitrogen into cyclic structures is therefore a timely and significant goal.
Among these methods, nitrosation is one of the most useful approaches to introduce nitrogen atoms to organic molecules. Common nitrosating reagents are mainly the salts and the esters of nitrous acid (HNO2), which are downstream products of nitric oxide (NO). NO, mostly known as a pollutant associated with exhaust fumes from combustion engines, is also industrially obtained in large quantity. Common nitrosating reagents are known for their deleterious environmental impact with the extensive generation of waste products.
Dark blue dinitrogen trioxide (N2O3) is a powerful nitrosating agent, which can be prepared from the reaction between NO and oxygen (O2) under strictly controlled and low temperature conditions. However, N2O3 is not stable at room temperature: it equilibrates with several decomposition products (NO, NO2, and N2O4), the utility of which is much lower.
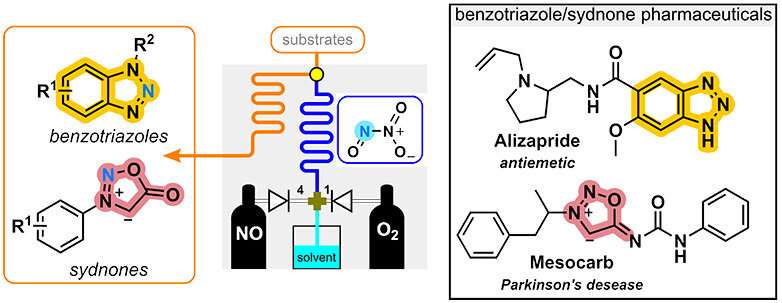
This instability is exacerbated when both liquid and gaseous phases are present, making the concentration of N2O3 in the liquid phase extremely difficult to control. Therefore, the applications of N2O3 in synthetic organic chemistry and the preparation added-valued N-heterocycles have been barely reported so far.
Attracted by the unique reactivity of N2O3 and the possibility to prepare it from a common pollutant (NO), researchers at the CiTOS laboratory (Center for Integrated Technology and Organic Synthesis) of the University of Liège (Belgium) led by Jean-Christophe Monbaliu, sought to develop a concrete solution for making N2O3 an accessible reagent for chemical synthesis.
The team relied on the inherent features of micro- and mesofluidic reactors for providing a unique technology-driven answer. "The elimination of the gas phase is permitted by continuous flow operation in the confined internal volume of micro and mesofluidic reactors," explains Yuesu Chen, first author and lead postdoctoral researcher affiliated with CiTOS.
"The accurate combination of NO and O2 in the presence of a suitable solvent forces N2O3 to be formed directly in the liquid phase, thus avoiding any deleterious side reaction and decomposition in the gas phase. A reliable and robust chemical generator of N2O3is therefore now available to the scientific community".
The upstream chemical generator of N2O3 feeds a downstream module for nitrosation reactions, therefore enabling its direct utilization for the preparation of a large diversity of unique N-heterocycles including benzotriazoles and sydnones, two specific backbones common to some pharmaceuticals.
"This research is of both practical and theoretical significance in organic nitrosation chemistry and the chemistry of nitroxides. It represents a significant breakthrough toward the development of modular continuous flow strategies for the preparation of libraries of valuable N-heterocyclic compounds. It simply unlocks the synthetic chemistry of N2O3, an unstable chemical difficult to tame otherwise," says Jean-Christophe Monbaliu.
More information: Yuesu Chen et al, On Demand Flow Platform for the Generation of Anhydrous Dinitrogen Trioxide and Its Further Use in N ‐Nitrosative Reactions, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202210146
Journal information: Angewandte Chemie International Edition
Provided by University de Liege