This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
An evolutionarily conserved pathway that achieves a peaceful co-existence with genomic parasites
Transposable elements are mobile genetic elements that can relocate within the genome and disrupt the normal function of genes, but are at the same time a source of evolutionary diversity. The lab of Tugce Aktas at the Max Planck Institute for Molecular Genetics has identified a novel pathway that keeps the activity of transposons in somatic cells in check after they have been transcribed.
Their findings have now been published in Nature. The work is a collaboration with the labs of Zachary D. Smith at the Yale Stem Cell Center, U.S., and Franz-Josef Müller from the Universitätsklinikum Schleswig-Holstein, Germany.
Over the course of evolution, the genomes of many organisms have become cluttered with ancient genetic remnants from evolution or parts of retroviruses that inserted their genetic code millions of years ago. Nearly half of the human genome consists of these transposable elements, or transposons.
And our genome has not stopped evolving. More like a work-in-progress document than static code, it is constantly changing its structure and sometimes even its content. Transposable elements can "jump" between and within genes, often by encoding proteins that facilitate their movement and by hijacking the cell's transcription machinery.
This obviously poses a threat by introducing mutations, but it is also a source of potentially beneficial variation in genes, offering potential for evolution and adaptation to environmental changes.
A fragile balance
Because transposons are both a curse and a blessing, organisms and transposable elements maintain a fragile balance for their continued coexistence. In the germline, where mutations can be passed on to offspring, systems are known to keep transposons transcriptionally silent and prevent their excessive activity.
"In somatic cells, transposons have been understudied because they cannot be inherited and their mobility is much more restricted," says group leader Tugce Aktas. "However, the fact that they are less mobile and studies linking their activity to disease suggest that there must be mechanisms that keep them in check.
"In our study, we have now discovered an evolutionarily conserved pathway that uses the RNA processing machinery to limit the negative effects of transposable elements."
A new layer of defense
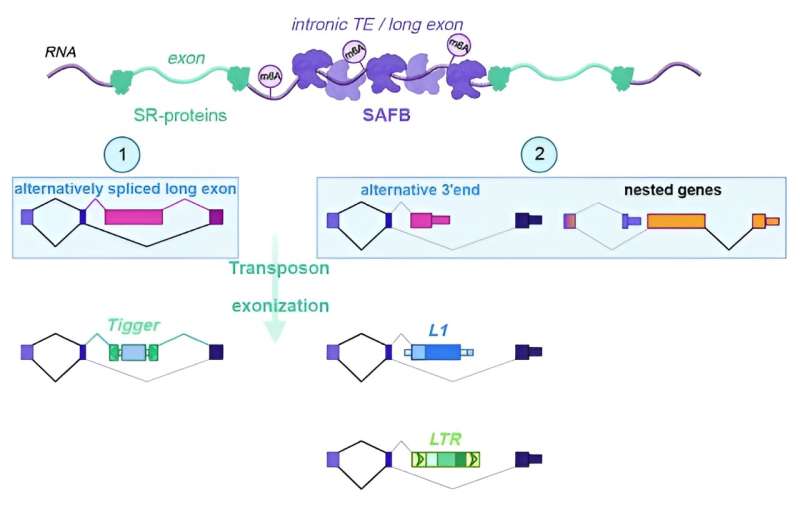
Before transcribed mRNA can be used to make proteins, premature mRNA must be cleared of introns in a process known as splicing. Unlike exons, these elements do not code for amino acids. Transposons share an evolutionary history with introns, and about 65% of human transposons are intronic. However, when they accidently end up within genes, they can alter gene expression and RNA processing, becoming "exonized" and thus part of a mature mRNA strand.
The scientists focused their research on L1, a common transposable element in the human genome, and tested its interactions with a group of proteins known to play important roles in splicing. They discovered that three so called scaffold attachment factor B (SAFB) proteins binds specific sequences in these transposons.
"When we removed the proteins, these bound parts were spliced in and became part of mature mRNA molecules," explains postdoctoral researcher Petar Glazar, one of the study's first authors. Thus, SAFB proteins effectively prevent the movement of L1 elements by sequestering their RNA products in the nucleus, where they are later degraded.
"Most known mechanisms focus on transcriptional silencing of transposons," says postdoctoral researcher Ibrahim Avsar Ilik, co-first author of the study. "Our work uncovers a new level of regulation of transposons at the post-transcriptional level. This is a striking discovery because it shows how an existing cellular pathway is repurposed to fight transposons, illustrating the evolutionary arms race between transposons and our genome."
Potential physiological relevance
The scientist's data show that the process is conserved and that the same families of proteins perform similar tasks in mice, flies and humans. The lab now aims to further characterize SAFB proteins, whose RNA-binding capabilities have been largely ignored.
At the physiological level, transposon activity in somatic cells has been linked to processes such as tissue and neuron formation, as well as disease. SAFB proteins are also an important component of nuclear stress bodies, enigmatic cellular structures that form under stress.
"Our goal now is to establish a collaboration with other experts to understand the biochemical and structural properties of SAFB proteins and their physiological relevance in tissues, especially in the brain," concludes Aktas.
More information: İbrahim Avşar Ilık et al, Autonomous transposons tune their sequences to ensure somatic suppression, Nature (2024). DOI: 10.1038/s41586-024-07081-0
Provided by Max Planck Society