This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Transmitting a domino reaction using redox chemistry achieved for the first time
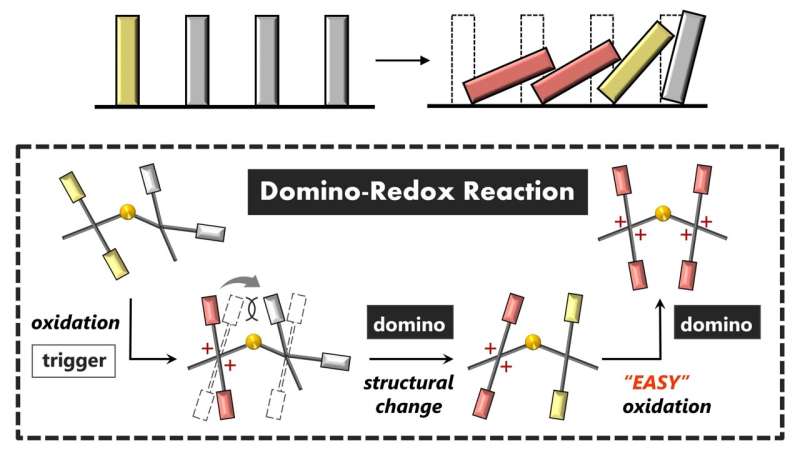
Domino reactions occur when the transformation of one chemical group stimulates the reaction of another attached group, or another molecule, leading to a rapid knock-on effect through the system like a row of falling dominoes. Researchers at Hokkaido University have now achieved the first example of a domino reaction in the branch of chemistry called redox chemistry.
The paper is published in the journal Angewandte Chemie International Edition.
The term redox comes from "reduction," referring to the gain of electrons, and "oxidation," referring to the loss of electrons. Redox reactions are therefore electron transfer processes.
"The problem with achieving domino reactions in redox processes is that the electron transfer, especially multi-electron transfer, produces electrically charged species whose electrostatic interactions can inhibit further change," says chemist Yusuke Ishigaki of the Hokkaido team.
To overcome the obstacles the researchers designed a two-part molecule that undergoes a significant structural change when one part is converted between its electrically neutral (reduced) and positively charged (oxidized) states. This structural change transmits a chemical effect to the other part of the molecule that makes its own oxidation more likely.
The molecule they designed consists of two relatively large redox-active units connected by a non-planar flexible link formed by sulfur atoms. When one of the paired units loses electrons (is oxidized), it acquires two positive charges which acts as the trigger causing the other part of the molecule to twist around the core. A change in the state of the electrons in this twisted form from the initial folded form then facilitates the oxidation process to occur in the neighboring group, achieving the domino effect.
The initial triggering of the reaction can be initiated by a temperature rise, offering a means of control. Although this effect has only so far been demonstrated within a two-part molecule, the researchers suggest it might eventually be used to transmit wave-like redox transformations in much larger molecules with many of the "domino" units linked together.
Applications of the discovery might be far in the future but there are clearly some general possibilities. Electrical and structural transformations traveling through molecular chains could become the nano-scale moving parts of chemical computation systems and sensors, for example. There are also possible applications in the new battery systems needed to support the ongoing transition to renewable electrical energy technologies.
"The control offered by heating and cooling could be used in many fields to make novel materials with switchable electronic properties, especially those involving multi-electron transfer," says Ishigaki.
"It was very challenging, but also very satisfying, to demonstrate what nobody had achieved before, and we now hope to move into larger and more complex systems involving increased electron transfer," Ishigaki concludes.
More information: Takashi Harimoto et al, Domino‐Redox Reaction Induced by An Electrochemically Triggered Conformational Change, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202316753
Journal information: Angewandte Chemie International Edition
Provided by Hokkaido University