This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A first for ferrocene: Organometallic capsule with unusual charge-transfer interactions
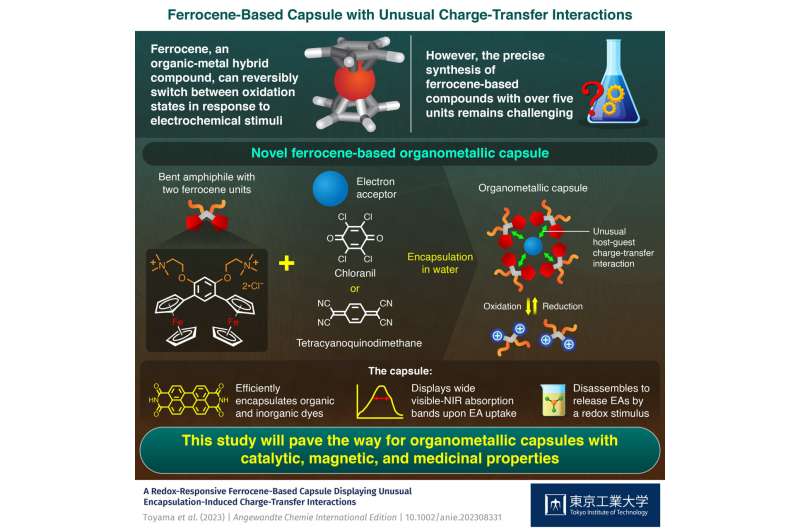
Ferrocene is an emergent organic-metal hybrid compound, and its accidental discovery has led to the rapid development of organometallic chemistry. Aside from its interesting structure, which consists of an iron atom sandwiched between two pentagonal organic rings, ferrocene has remarkable redox-responsive properties.
Simply put, one can make ferrocene-based compounds reversibly switch between different oxidation states by changing the conditions of their redox environment, which essentially dictates how electron transfers occur between molecules. Although the properties of ferrocene-based compounds could be very useful in materials science, drug delivery, and catalysis, there are almost no known methods to facilely and precisely synthesize multi-ferrocene-based capsules with more than five ferrocene units.
Fortunately, a research team from Tokyo Institute of Technology in Japan has found a solution to this problem. In their latest study, which was conducted by Kazuki Toyama (doctoral student), Assistant Professor Yuya Tanaka, and Professor Michito Yoshizawa, and published in Angewandte Chemie International Edition, the researchers found an ingenious way to prepare a ferrocene-based capsule with unusual properties.
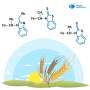
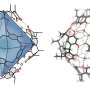
The key to the synthesis of the capsule is a ferrocene-containing amphiphile, which the researchers referred to as "FA". Each FA molecule consists of two hydrophobic ferrocene groups bound to a meta-phenylene ring, which is also connected to two hydrophilic trimethylammonium groups. The bent hydrophobic framework and its hydrophobic effect cause multiple FA molecules to quickly and spontaneously assemble into organometallic capsule (FA)n in water.
Interestingly, the capsule can be reversibly disassembled and assembled by providing appropriate chemical stimuli. For example, adding an oxidant such as iron chloride to water containing (FA)n leads to the immediate disassembly of the capsule. Moreover, the subsequent addition of a reductant such as ascorbic acid neutralizes the oxidant, leading to the quick reassembly of the capsule.
The on-demand assembly and disassembly of the new capsule becomes even more useful when considering that it can bind to guest molecules in the cavity. The researchers found that capsule (FA)n is a more versatile host than previous ones, as Toyama says, "In contrast to previous multi-ferrocene compounds, the present capsule efficiently encapsulates typical organic and inorganic dyes, such as perylenetetracarboxylic diimide and copper-phthalocyanine, as well as electron-accepting molecules, such as chloranil and tetracyanoquinodimethane, in water."
The team discovered unusual host-guest charge-transfer interactions upon the encapsulation of electron-accepting molecules by the capsule (FA)n. The interactions were observed as relatively wide absorption bands, ranging from 650-1350 nm, in the visible to near-infrared spectrum. These interactions could also be reversibly turned on and off by controlling the assembly and disassembly of the capsule.
The present multi-ferrocene-based capsule could find applications across various fields, such as medicine, biotechnology, chemical synthesis, and more. Further studies are already underway. "On the basis of the present achievement, our next study will focus on the development of various types of organometallic capsules, such as ones with magnetic and medicinal properties and catalytic activity," concludes Dr. Tanaka.
More information: Kazuki Toyama et al, A Redox‐Responsive Ferrocene‐Based Capsule Displaying Unusual Encapsulation‐Induced Charge‐Transfer Interactions, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202308331
Journal information: Angewandte Chemie International Edition
Provided by Tokyo Institute of Technology