This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study finds S. aureus' surface-sticking ability not evenly distributed over cell envelope
Infections caused by the bacterium Staphylococcus aureus have a significant impact on human health, with tens of thousands of hospital patients dying every year from infections due to the S. aureus "superbug." Stopping the spread of bacteria like S. aureus will require not only the development of new antibiotics to which antimicrobial resistance has not been established, but also a better understanding of how these germs adhere to surfaces and from where they can enter the human body. In hospitals, surfaces that may be infected with S. aureus include catheters and implants.
A research team led by Professor Karin Jacobs at Saarland University and Professor Markus Bischoff at Saarland University Medical Center has developed an innovative approach that has enabled them to unravel the secrets of bacterial adhesion. Their study is published in the journal Soft Matter.
Via a technique known as single cell force spectroscopy (SCFS), a single living bacterium is attached to a tiny spring-like tip called a cantilever. The cantilever with bacterium attached is then pressed gently onto a surface. The cantilever is subsequently retracted and the force required to detach the bacterium from the substrate measured. The force involved is only a few nanonewtons—equivalent to a billionth of the weight of a bar of chocolate.
Earlier studies by the team showed that the contact area between the bacterium and the substrate has a diameter of 150–300 nanometers, i.e., between about a third and a sixth of the diameter of the S. aureus cell. Conclusions about the adhesion force could therefore only be made in relation to this limited range.
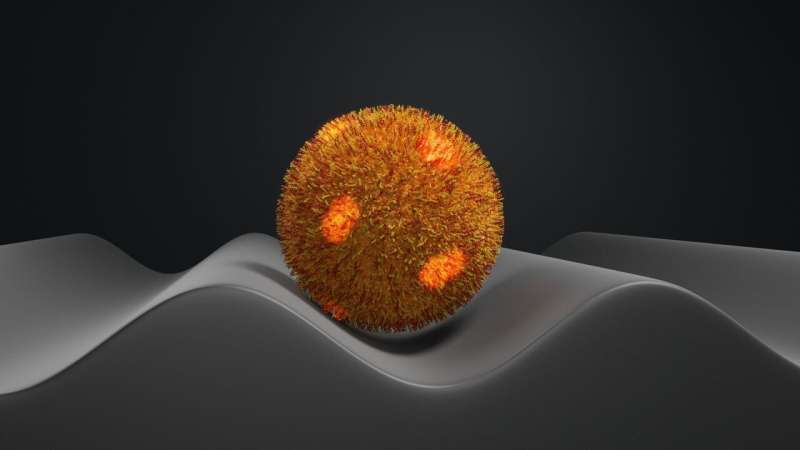
In the present study, the bacterium was not placed on a planar substrate, but on a sinusoidally corrugated surface. By recording the force-distance curve over this "wrinkled" surface, the researchers were able to map out the strength of the adhesion force over almost the entire lower half of the bacterium. The substrate was provided by research partners at Dresden University of Technology.
The results were striking. The adhesion force was found to vary significantly from cell to cell because the force of adhesion is not evenly distributed over the bacterial cell envelope and because each bacterium is held by the cantilever in a particular fixed position on the wrinkled surface.
To gain a better understanding of the nature of these highly adhesive patches, Dr. Michael Klatt developed a number of geometric models of the bacterial surface in order to find the one that best matched the results of the experimental force-distance curves, which show the force that has to be applied to detach the bacterium from the substrate. The model that proved best able to reproduce the experimental results had three to six adhesion sites, each with a diameter of about 250 nm, that were distributed over the cell envelope as widely as possible.
The experiments also showed that even in the absence of the highly adhesive patches, the adhesion force recorded in the surface minima ("valleys") is about twice as strong as in surrounding areas. It is notable just how rapidly the adhesion force decreases as a cell moves out of the valley. To gain greater insight into the experimental data, numerical simulations ("Monte Carlo simulations") were carried out by Dr. Erik Maikranz.
The simulations showed that the force required to detach the cell from the surface not only depends on the contact area between the bacterium and the surface but is also strongly influenced by the angle at which the adhesion force between the bacterium and the substrate acts.
This angle depends on where exactly the bacterium is located on the corrugated surface structure. If it sits on a peak ("surface maximum"), the contact area is small and the force required to detach the bacterium from the surface is therefore also usually small, unless the peak of the surface happens to be in contact with one of the bacterium's highly adhesive patches.
The area of contact is larger if S. aureus is located on one side of the valley wall, but the angle of interaction in this case is also large, so that the vertical component of the adhesion force acting between the cell and the substrate is still low. It is this vertical component of the adhesion force that SCFS measures. The detachment force is far greater when the bacterium sits on the "valley floor." In this location, the contact area is large, as the curvatures of bacterium and valley match, but the angle of interaction is now small again, so that most of the adhesion force acts vertically.
The results of the Saarbrücken study therefore offer insight into why the adhesion force exhibited by bacteria of the same species on the same substrate material can vary so greatly from cell to cell. Using this new approach, it is now possible to specify not only a typical value of the adhesion force, but how this force varies across the surface of the bacterial cell and how a structured substrate influences adhesion.
However, the question of how these locally increased adhesion forces are created at the molecular level within the highly adhesive patches in the bacterial cell wall has not been fully resolved. It may in part be due to the presence of clusters of adhesins, which are components on the cell surface that facilitate adhesion of the bacterium to the substrate. For a bacterium such as S. aureus, this has the advantage of creating a very adhesive surface without requiring significant biosynthetic effort on the part of the pathogen.
If such a highly adhesive patch comes into contact with the substrate surface, the probability that the bacterium will "stick" to the surface increases. This could be particularly advantageous for spheroidal bacteria that can "roll" over the substrate surface even under low flow conditions. This would increase the chance that one of these strong adhesion sites comes into contact with the substrate, thus anchoring the bacterium to the surface.
This advantage may well be magnified on living substrate surfaces, as the bacterium can dock onto ligands on the surface of the host tissue or biomaterial, thus enabling a particularly strong interaction.
The findings from this basic research study have potentially important implications for the development of new materials and the design of future studies on bacterial adhesion. They also open up new possibilities in the field of biomedical research and could ultimately help to significantly reduce infections caused by catheters and medical implants.
More information: Christian Spengler et al, The adhesion capability of Staphylococcus aureus cells is heterogeneously distributed over the cell envelope, Soft Matter (2023). DOI: 10.1039/D3SM01045G
Journal information: Soft Matter
Provided by Saarland University





















