This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists find malaria parasite takes its time within mosquito host to reap an evolutionary advantage
The malaria parasite is a master of adaptation. To complete its life cycle, the parasite must be transmitted from a mosquito to a human and then back to a mosquito again. Over millions of years of evolution, it has adapted perfectly to these two hosts. But although we have known about the malaria cycle for over 100 years, many questions remain unanswered.
One of these questions concerns the duration that malaria parasites need to develop inside the mosquito. It is currently estimated that malaria parasites can be transmitted after an average of 12 days. However, mosquitoes are expected to live on average two weeks in nature.
Therefore, the 12 days required for their development seems counterintuitive: One would expect the parasite to be transmitted to a human as soon as possible, because every day the parasite spends in a mosquito increases the chance that the mosquito will die before the parasite is transmitted by a bite. So, what is the advantage that justifies the risk of not being transmitted?
Evolutionary compromise
Researchers of the Levashina Lab at the Max Planck Institute for Infection Biology in Berlin have now uncovered this advantage—and created the basis for a better understanding of parasite evolution in the mosquito. They showed that parasites benefit from the mosquito's rich supply of nutrients if they stay there longer before being transmitted to a human, ultimately leading to a more successful transmission. The time at which a parasite is transmitted to a human is therefore an evolutionary trade-off between "getting fit for infection" and "leaving on time."
The paper is published in the journal Nature Communications.
Based on findings from laboratory experiments, these results were obtained by employing mathematical models. "It is virtually impossible to follow the evolution of malaria parasites in the laboratory," explains Paola Carrillo-Bustamante, first author of the study. "To study the evolutionary pressures, one would need to follow hundreds of transmission cycles: mosquito-mammal-mosquito. This is impossible for human malaria parasites."
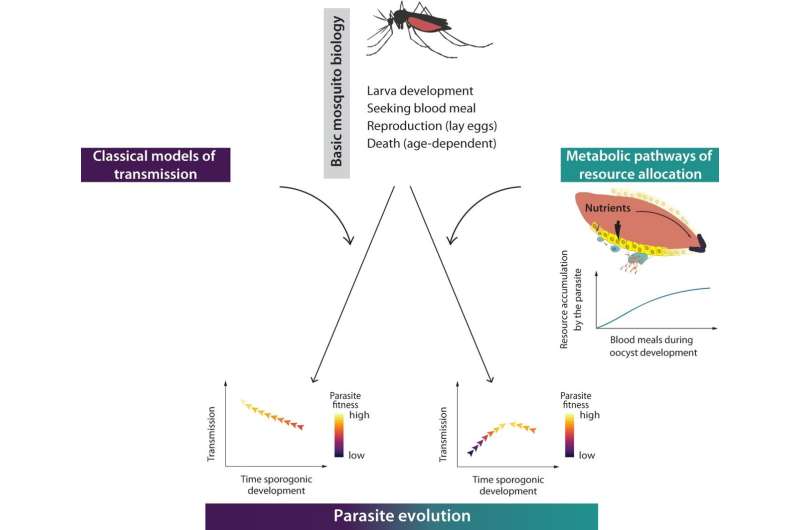
Mathematical models capture real processes in mathematical formulae, allowing researchers to make predictions and to analyze and understand the behavior of these processes under different conditions. There are already models of malaria transmission that basically describe the life of the mosquito. However, they usually only include variables for larval development, biting behavior, reproduction and the age of the mosquitoes. These models predict the intuitive expectation that malaria parasite would evolve a short development period, which, as described above, is not the case in reality.
Optimized model
The fact that models do not fully correspond to reality is not in itself a problem, as they are always only an approximation of reality. However, if a model does not correctly represent key mechanisms, it needs to be supplemented. The researchers' task was to find the missing variable that would allow a transmission model to correctly describe the development time of the parasites. Previous studies of mosquito metabolism provided clues to the missing variable.
To develop their eggs, female mosquitoes need nutrients from the blood of mammals, this is why they bite humans. During this blood-meal, malaria parasites enter the mosquito gut, and use the same nutrients for their own development. However, a mosquito often bites more than once in its lifetime.
"Our model predicts that the parasite becomes stronger with each blood meal, so it benefits from multiple bites," says Carrillo-Bustamante. "With this crucial information, we revised the transmission model. Multiple bites take time—a possible reason why the parasite waits a long time inside the mosquito to benefit from as many blood meals as possible."
More than just a syringe
Adding the new variable "metabolism," the researchers then carried out evolutionary experiments with the updated transmission model. They gave virtual parasites the opportunity to randomly change their development time in the mosquito, i.e., to mutate. They then allowed the model to run for 5,000 days—enough time for natural selection to find an optimal development time.
Regardless of whether the starting point was a short or long developmental time parasites with a development time of 12 days always got selected in the population. This evolutionary optimum can also be observed in reality. For Carrillo-Bustamante, the result indicates that existing models need to be supplemented. "Previous models have often treated the mosquito-like a syringe that transmits the malaria parasite to humans. Our study shows that the mosquito-parasite interactions must be considered in transmission models."
Malaria remains a moving target. We need to understand the disease in all its facets to develop effective countermeasures, as 250 million people still are infected with malaria every year. Accurate models of disease transmission are an important step in understanding the disease and correctly predicting future epidemics. This is particularly important in an era of changing environmental conditions, as climate change brings malaria to new regions of the world.
More information: Paola Carrillo-Bustamante et al, Evolutionary modelling indicates that mosquito metabolism shapes the life-history strategies of Plasmodium parasites, Nature Communications (2023). DOI: 10.1038/s41467-023-43810-1
Journal information: Nature Communications
Provided by Max Planck Society