This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New research examines corrosion on atomic level
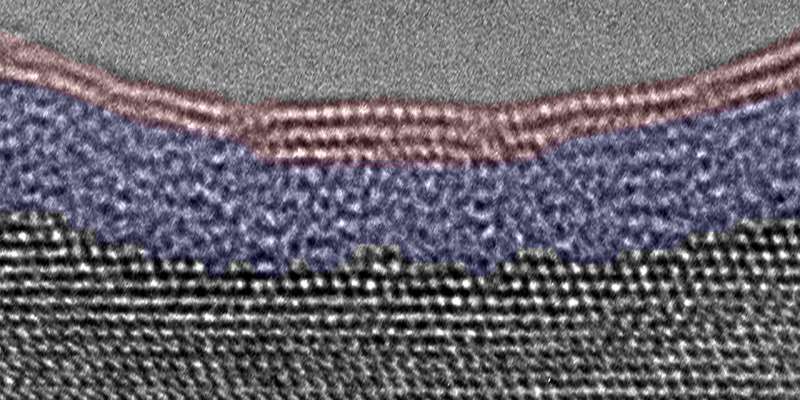
When water vapor meets metal, the resulting corrosion can lead to mechanical problems that harm a machine's performance. Through a process called passivation, it also can form a thin inert layer that acts as a barrier against further deterioration.
Either way, the exact chemical reaction is not well understood on an atomic level, but that is changing thanks to a technique called environmental transmission electron microscopy (TEM), which allows researchers to directly view molecules interacting on the tiniest possible scale.
Professor Guangwen Zhou—a faculty member at Binghamton University's Thomas J. Watson College of Engineering and Applied Science—has been probing the secrets of atomic reactions since joining the Department of Mechanical Engineering in 2007. Along with collaborators from the University of Pittsburgh and the Brookhaven National Laboratory, he has studied the structural and functional properties of metals and the process of making "green" steel.
Their latest research, "Atomistic mechanisms of water vapor induced surface passivation," was published in November in the journal Science Advances.
In the paper, Zhou and his team introduced water vapor to clean aluminum samples and observed the surface reactions.
"This phenomenon is well-known because it happens in our daily lives," he said. "But how do water molecules react with aluminum to form this passivation layer? If you look at the [research] literature, there's not much work about how this happens at an atomic scale. If we want to use it for good, we must know because then we will have some way to control it."
They discovered something that had never been observed before: In addition to the aluminum hydroxide layer that formed on the surface, a second amorphous layer developed underneath it, which indicates there is a transport mechanism that diffuses oxygen into the substrate.
"Most corrosion studies focus on the growth of the passivation layer and how it slows down the corrosion process," Zhou said. "To look at it from an atomic scale, we feel we can bridge the knowledge gap."
The cost of repairing corrosion worldwide is estimated at $2.5 trillion a year, which is more than 3% of the global GDP—so developing better ways to manage oxidation would be an economic boon.
Additionally, understanding how a water molecule's hydrogen and oxygen atoms break apart to interact with metals could lead to clean-energy solutions, which is why the U.S. Department of Energy funded this research and Zhou's similar projects in the past.
"If you break water into oxygen and hydrogen when you recombine it, it's just water again," he said. "It doesn't have the contamination of fossil fuels, and it doesn't produce carbon dioxide."
Because of the clean-energy implications, the DOE has regularly renewed Zhou's grant funding over the past 15 years.
"I greatly appreciate the long-term support for this research," Zhou said. "It's a very important issue for energy devices or energy systems because you have a lot of metallic alloys that are used as structural material."
More information: Xiaobo Chen et al, Atomistic mechanisms of water vapor–induced surface passivation, Science Advances (2023). DOI: 10.1126/sciadv.adh5565
Journal information: Science Advances
Provided by Binghamton University



















