This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Revolutionizing strawberry production: CRISPR/Cas9 editing enhances fruit firmness and extends shelf life
The cultivated strawberry (Fragaria × ananassa, Duch.) is one of the Rosaceae family renowned for its flavor and health benefits, making it an important agricultural commodity. However, its soft texture leads to a brief shelf life and consequent economic losses.
Current breeding aims to enhance firmness without compromising quality, but the achievements have been limited. The softening of the fruit is accompanied by the disassembly of cell walls and the middle lamella during ripening. Pectin is the cell wall component that undergoes the most extensive changes during strawberry softening.
Research has identified that pectinase enzymes, especially polygalacturonases (PGs), are instrumental in this softening process. While traditional breeding methods are slow, especially due to the high ploidy of strawberries, modern methods such as CRISPR offer precision and speed. The current research gap lies in expanding the use of CRISPR to include a wider range of agronomic traits in cultivated strawberries, potentially revolutionizing their production.
In February 2023, Horticulture Research published a research paper entitled "CRISPR/Cas9 editing of the polygalacturonase FaPG1 gene improves strawberry fruit firmness".
In this study, FaPG1 knockout strawberry plants were generated using the CRISPR/Cas9 system. The genomic sequence of FaPG1 was cross-referenced with the most recent Fragaria × ananassa genomes. Two annotations were located on chromosome 6A, one in the Camarosa genome (FxaC_21g15770) and the other in the Royal Royce genome (Fxa6Ag103973).
The FxaC_21g15770 sequence was used to select an sgRNA for editing, with its target site in the first exon encoding part of the glyco hydro 28 domain. No allelic variation was found in this region. FaPG1 homoeologous were identified in the Royal Royce genome, all exhibiting a specific deletion near the mutation target site.
The chosen sgRNA was cloned and transferred to the pDe-CAS9 vector, which provides resistance to phosphinothricin in plants. Transformation was carried out using Agrobacterium tumefaciens on "Chandler" strawberry plants, yielding over 15 resistant lines.
Ten of these were assessed, and all showed successful FaPG1 editing via the T7 endonuclease I assay. In-depth sequencing revealed editing efficiencies ranging from 47% to nearly 100% across the assessed lines. Analysis of the mutation events disclosed 14 distinct edited sequence combinations, with deletions being predominant. Each plant line exhibited varied combinations of these mutation events. Amino acid sequences deduced from these events showed either frame-shifting, ORF preservation with amino acid loss, or residue substitutions.
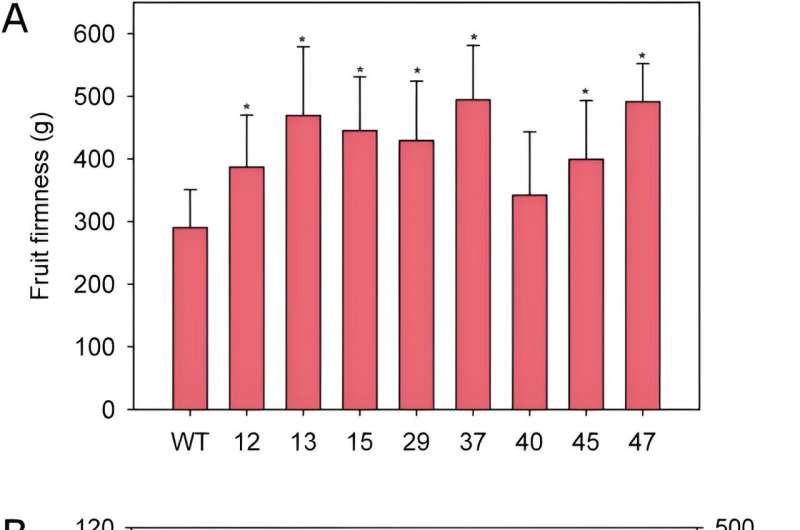
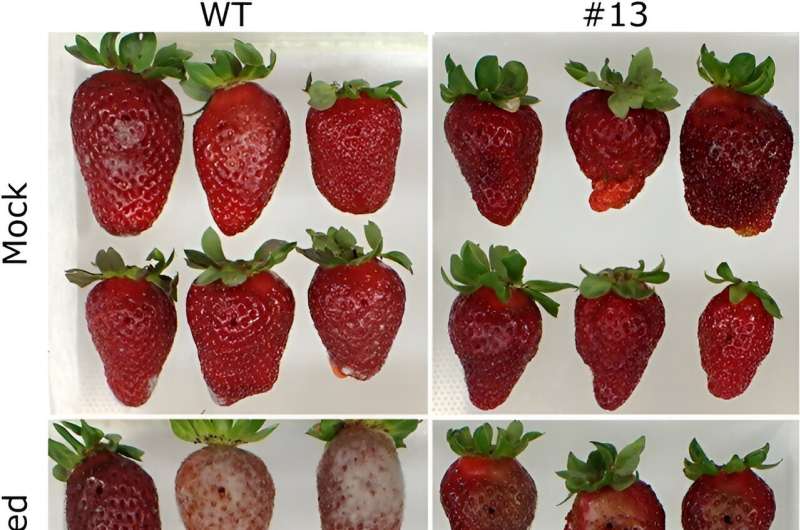
Phenotypically, edited plants showed similar vegetative growth to wild types. Fruit quality parameters of eight chosen lines revealed alterations in weight, length, color, and firmness, depending on the line, of which the ratio length/width was lower than the wild type in most lines, and transgenic fruits were less elongated and squarer when compared to the control. In addition, fruit firmness significantly increased in almost all edited lines, and there was a clear positive relationship between the degree of FaPG1 editing and fruit firmness at harvest.
When assessing post-harvest traits, edited fruits showed reduced softening rates and increased resistance to fungal rot compared to wild types. Researchers further studied fruit susceptibility to Botrytis cinerea, and the results showed an enhanced resistance in edited fruits. Potential off-target effects were investigated using the CRISPOR web tool, which identified four possible off-target genes. However, subsequent testing on the lines with the highest editing percentages revealed no off-target mutations.
In conclusion, researchers successfully edited the FaPG1 gene in strawberry plants using the CRISPR/Cas9 system delivered via Agrobacterium, reducing fungal susceptibility and transpirational water loss, globally resulting in improved fruit firmness and postharvest shelf life.
This study underlines the potential of gene editing to enhance the post-harvest qualities of strawberries, offering significant value for future agricultural applications.
More information: Gloria López-Casado et al, CRISPR/Cas9 editing of the polygalacturonaseFaPG1gene improves strawberry fruit firmness, Horticulture Research (2023). DOI: 10.1093/hr/uhad011
Journal information: Horticulture Research
Provided by NanJing Agricultural University