This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Reprogramming tissue mechanically to promote wound healing
Researchers at PSI and ETH Zurich have taken connective tissue cells that have been mechanically reprogrammed to resemble stem cells and transplanted them into damaged skin. In their laboratory experiment, they were able to show that this can promote wound healing.
Mature somatic cells can be turned back into youthful, stem-cell–like cells by means of a surprisingly simple mechanical stimulus. With the help of this method, researchers have now partially reprogrammed fibroblasts and successfully transplanted them into a model of old, damaged skin tissue in a laboratory experiment. They were able to show that the skin tissue model can actually be rejuvenated and that injuries heal better.
The researchers led by G.V. Shivashankar, head of the Laboratory for Nanoscale Biology at PSI and Professor of Mechanogenomics at ETH Zurich, have now published their findings in the journal Aging Cell.
Simple trick for better wound healing
Fibroblasts are not yet fully differentiated cells, meaning that they can develop into various different types of connective tissue. They play an important role in skin regeneration and wound healing. Shivashankar and his team succeeded in turning these fibroblasts back into partially stem-cell–like cells.
Unlike true, so-called pluripotent stem cells, which can develop into almost any type of cell, the stem-cell–like fibroblasts are restricted to connective tissue. Nevertheless, they are in a more fundamental state and have even more different ways of developing than the actual fibroblasts. What makes the technique so special is that the researchers didn't use genetic engineering or chemicals but reprogrammed the cells entirely through mechanical stimulation.
To do this, they first embedded the fibroblasts in a matrix made of fibronectin, a protein to which the cells can attach themselves. The narrow mesh of the matrix means that there is only room for about four fibroblasts per enclosure during cell division. If the fibroblasts continue to divide, they are forced to spread out in the third dimension, i.e., upwards.
"The amazing thing," says G.V. Shivashankar, "is that the information stored about their form and function appears to get lost during this transition. In a sense, they forget what they were originally there for." Hence the fibroblasts become stem-cell–like cells simply by growing under spatially confined conditions.
"It's important to choose the matrix density such that the original cell is not squeezed together, because in that case the cell would die," Shivashankar continues. "The cell must only encounter the barrier once it divides; then it will transform." And the tests show that it does this extremely efficiently. The researchers obtained a large number of stem-cell–like fibroblasts with comparative ease. Shivashankar and his colleagues published these interim achievements in 2018 and 2020.
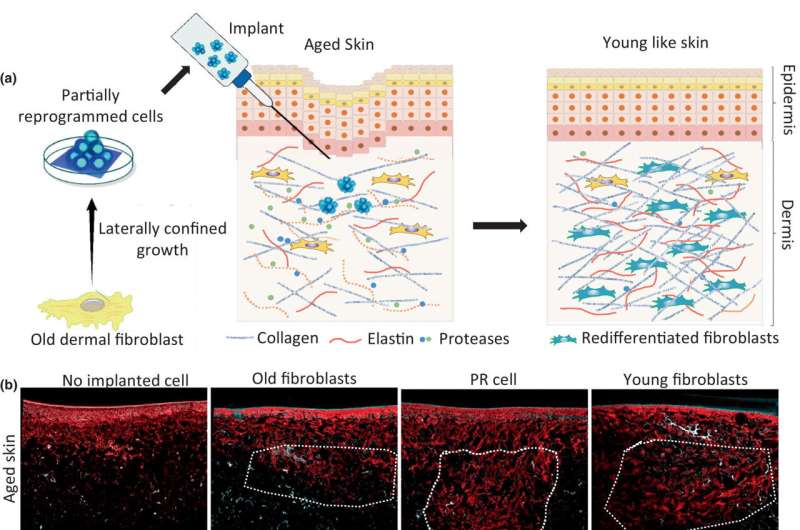
The present study builds on their previous triumph. In their experiment, the researchers took aged cells from real skin, reprogrammed them to become stem-cell–like fibroblasts using their technique, and then inserted them into a model of old, injured skin tissue in the laboratory.
"The cells began to produce more proteins again in order to form new skin. Regeneration and wound healing were considerably faster compared with the transplantation of unmodified cells," reports Shivashankar. This was because reprogramming also erased the functional errors accumulated by the old cells during the aging process. Just as reformatting a hard drive makes it faster again when running newly installed programs.
An alternative to established skin transplants
The group's research is motivated by the fact that current therapies for extensive skin injuries are limited. So-called cell-based therapies are used on burns, for example: here healthy tissue is removed from other areas of the patient's body and grafted onto the injured region. Alternatively, cell tissue from another person can also be transplanted. However, both methods have their limitations. Tissue transplanted from a donor can trigger a rejection reaction. And with elderly people, it is often difficult to obtain enough of the patient's own skin.
Partially reprogrammed stem-cell–like fibroblasts provide a solution. Their distinguishing feature is that they are undifferentiated, in a youthful state, so to speak. Depending on the environment in which they find themselves, they mature to become different types of cells, including skin cells.

Reprogramming without genetic engineering
The idea of reprogramming cells dates back to 2006. At the time, Japanese researcher Shinya Yamanaka found a way of genetically manipulating mature cells to turn them back into stem cells. The discovery caused a sensation, because until then this was believed to be impossible. So far, stem cells have been extracted from the bone marrow or blood of donors in order to treat blood cancer, for example.
However, Yamanaka discovered four genes that trigger the reprogramming of a cell: the so-called "Yamanaka factors." When these are implanted into a cell, it becomes what has since become known as an iPS cell (induced pluripotent stem cell). In 2012, Yamanaka was awarded the Nobel Prize in Medicine for his discovery.
Since then, numerous teams around the world have been investigating how iPS cells can be used in cell-based therapy and whether there are other ways of reversing their development aside from genetic engineering. Genetic manipulation remains ethically controversial. It has also been shown that iPS cells have a tendency to proliferate like tumors.
Some research groups are working on preventing this side effect. Others are studying biochemical methods instead of genetic engineering; here the transformation into stem cells is triggered by introducing special molecules. Shivashankar's group at PSI is, in turn, the world leader in mechanical reprogramming.
Of interest to medicine and cosmetics
One of the questions currently being investigated by the PSI group is the precise mechanisms that cause reprogramming as a result of confinement. For many years, Shivashankar and his team have been investigating how cell geometry is linked to gene expression.
Depending on how the DNA is packed, and possibly constricted, inside a cell's nucleus, it may be impossible to read certain genes, which in turn results in certain diseases. In the course of these investigations, the group has trained a computer program using artificial intelligence to recognize the corresponding features in the images of cell nuclei, thereby improving the early diagnosis of diseases.
To round off their current research findings on wound healing, the team is now planning to conduct experiments on real human skin that has not been grown in a laboratory. Shivashankar is convinced that they will be able to replicate their previous success.
Moreover, these findings will not only benefit medical applications. "Cosmetic applications are also conceivable," says Shivashankar, "because, in principle, we can make new tissue out of old." Apart from skin tissue, regenerating muscle or brain cells is also conceivable. "In any case, the method has the potential to allow us to age more healthily."
What's more, the technique is so straightforward that, in principle, any medical student can apply it. And it is in line with the general trend towards personalized medicine, in which substances are tailored to the individual patient. In this case, the cells are actually the patient's own and no foreign materials are introduced at all.
After the first paper was published, several pharmaceutical companies already expressed an interest in refining the process. Although it will take a few years before clinical applications emerge, Shivashankar says, "We are really excited about where this research will lead us."
More information: Bibhas Roy et al, Implanting mechanically reprogrammed fibroblasts for aged tissue regeneration and wound healing, Aging Cell (2023). DOI: 10.1111/acel.14032
Journal information: Aging Cell
Provided by Paul Scherrer Institute