This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Early epigenetic instructions anticipate next steps of gene activity during blood cell development
University of Birmingham researchers are working to understand how cells are prompted to develop into different cell types, vital for performing different functions within the body. In this study published in Life Science Alliance, researchers were looking at the differentiation of early cell types, known as embryonic stem cells, into blood.
This study builds on work from a previous Nature Communications publication earlier this year, where the team led by Professor Constanze Bonifer and Professor Jean Baptiste Cazier, developed a new test that functionally identifies all regions in the genome that are responsible for activating genes during the early stages of blood cell development, called enhancers.
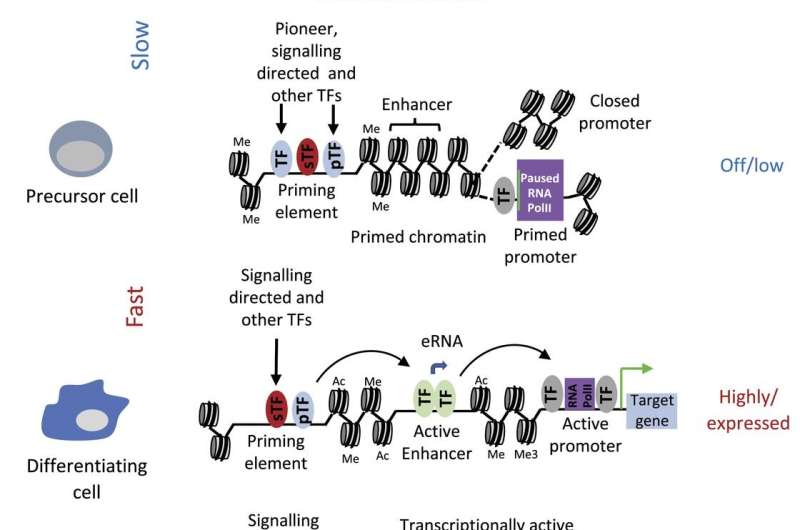
More recently, they have studied the activity of these DNA elements in detail and found that many of them are already activated way before the start of gene expression, a process called "chromatin priming." Chromatin is a mixture of DNA and proteins, where the proteins are responsible for packaging the DNA into a unit capable of fitting inside a cell nucleus. Chromatin can be referred to as either "open" or "closed."
This recent study has identified regulatory elements that exist in open chromatin before the onset of expression of their linked gene. Essentially, at each step of development, the next step is already being anticipated.
"Chromatin priming and the precise timing of the activation of enhancer elements driving gene expression at the right time and in the right cell type is at the very heart of all coordinated, synchronized cell differentiation processes that create fully developed multicellular organisms," says Bonifer.
The study used gene editing to take out one of these priming elements and showed that they play a very early role in the instruction booklet for cell development. Without them the process stalls before it starts. Moreover, they also found that outside signals can program the activity of priming elements, thus setting specific developmental pathways in motion.
Professor Jean-Baptiste Cazier, from the Institute of Cancer and Genomics, the University of Birmingham said, "Our priming element resource adds an additional dimension to our ability to interrogate the fine details of hematopoietic specification from embryonic stem cells and model blood cell development in vitro and in silico."
Cell development is a complex process with many steps and scientists have been working to understand the details of these processes for many years. Understanding how these complex processes work will give us a better understanding of when things go wrong, the knock-on effect that can cause, and may even shed light on how to target treatments at these malfunctioning processes to alleviate disease.
More information: Alexander Maytum et al, Chromatin priming elements direct tissue-specific gene activity before hematopoietic specification, Life Science Alliance (2023). DOI: 10.26508/lsa.202302363
B. Edginton-White et al, A genome-wide relay of signalling-responsive enhancers drives hematopoietic specification, Nature Communications (2023). DOI: 10.1038/s41467-023-35910-9
Journal information: Nature Communications
Provided by University of Birmingham