This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Will this antibiotic work for you? A quick and easy method of testing antimicrobial susceptibility
Antimicrobial resistance (AMR) directly causes an estimated 1.3 million deaths around the globe annually, a figure that the World Health Organization has predicted will rise. A leading cause of AMR is the misuse and overuse of antibiotics, which has allowed microbes to mutate over time and develop insensitivity to the drugs designed to kill them, making infections harder to treat and increasing the risk of disease spread.
A key step to preventing AMR is to test the susceptibility of a microbe against certain antibiotics before prescribing them to a patient, which increases the likelihood of effective treatment. While current methods of evaluating AMR exist, they are incredibly slow with a turnaround time of over 20 hours. This emphasizes the need for a rapid method that evaluates antimicrobial susceptibility without sacrificing accuracy.
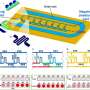
This is the challenge that Associate Professor Ye Ai from the Singapore University of Technology and Design's (SUTD) Engineering Product Development pillar chose to undertake. In a paper titled "Rapid and accurate antimicrobial susceptibility testing using label-free electrical impedance-based microfluidic platform" and published in Small, Assoc Prof Ai and his team successfully developed an electrical impedance-based microfluidic platform that provides rapid and accurate AMR evaluation within an hour.
Currently, broth microdilution and disk diffusion are the gold standard for evaluating AMR. However, these culture-based methods rely heavily on manual work and subjective visual measurement of bacterial growth that require at least 18 to 24 hours of incubation.
"As a result, this often leads to antibiotics being prescribed presumptively without a clear indication of their efficacy in vitro, potentially resulting in the prolonged or excessive use of broad-spectrum antibiotics," commented Assoc Prof Ai.
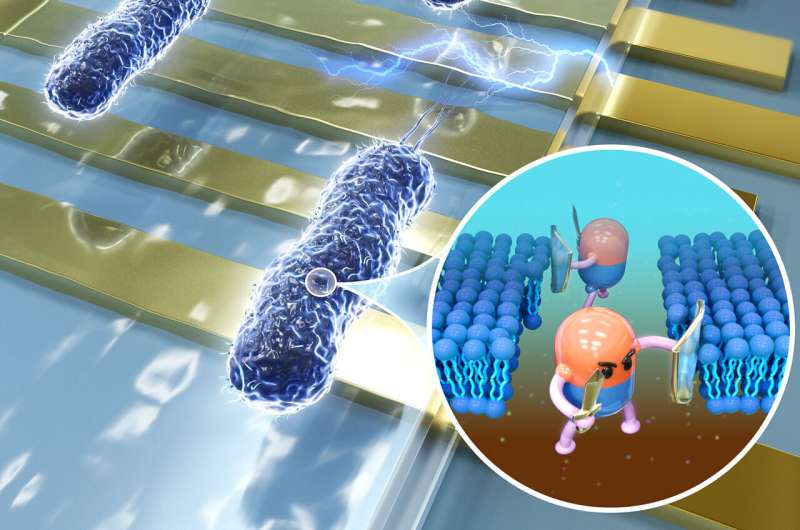
To combat this, the research team developed a novel electrical impedance-based microfluidic platform that can perform rapid antimicrobial susceptibility testing (AST). It works by measuring the bacterial membrane permeability after antibiotic exposure.
"Because the change in membrane permeability of susceptible bacteria can be detected by the impedance-based AST as fast as 30 minutes after exposure to antibiotics, long-term bacterial culture is not required in our approach," explained Assoc Prof Ai.
Doing away with the long-term bacterial culture eliminates the long waiting time that comes with it. Using the microfluidic platform can reduce the turnaround time for results by around 20 hours compared to culture-based methods. Moreover, the platform permits fully automated handling of bacterial solutions, reducing human error and increasing the reliability of AST results.
To test the accuracy of the platform, the research team conducted comparative analyses with culture-based methods. "This is a critical step to convince clinical laboratories and health care providers to integrate and test our innovative AST solutions in their existing diagnostic workflow," said Assoc Prof Ai.
First, the team determined the minimum inhibitory concentration (MIC) for three different E. coli strains (Eco1, Eco2 and Eco3) at different concentrations of the antibiotic ciprofloxacin. The microfluidic platform indicated the following MICs: Eco1 4 mg/L.
Since CLSI guidelines state that bacteria with MIC less than 0.25 mg/L is susceptible to a certain antibiotic and MIC more than 1 mg/L is resistant to it, the results showed that Eco1 and Eco2 strains are susceptible to ciprofloxacin while Eco3 strain is resistant. These findings were in full agreement with the results from the broth microdilution method.
Second, the team evaluated the breakpoints for different antibiotics. Breakpoints indicate drug potency against potential pathogens, the pharmacokinetics and the pharmacodynamics of the drug, and drug dosage regimens to be used in the clinic. Here, the team tested the microfluidic platform to see if it can rapidly measure the breakpoints of ciprofloxacin and classify pathogenic strains as susceptible or resistant. Again, the platform was able to differentiate the strains accurately, and its results also fully agreed with the results of the disk diffusion test.
Finally, the team checked if the platform can be used in a broader manner. Eco1, Eco2 and Eco3 were treated with different antibiotics to test if the platform can successfully differentiate between susceptible and resistant strains, depending on the antibiotic. For verification, the disk diffusion method was done in parallel. Results once again showed that the microfluidic technology can correctly identify which strains are susceptible to which antibiotics.
In all tests, the electrical impedance-based microfluidic platform yielded 100% categorical agreements compared to the gold-standard AMR evaluation methods, making it a highly promising AST tool. The implications of this technology are huge. With a fast and accurate way of checking if a certain antibiotic will work against a specific microbe, the threat of AMR is greatly mitigated.
In the hopes of translating this technology into a real product to combat AMR, Assoc Prof Ai is actively looking for commercialization funds to bring this technology to the wider market and is also working with Changi General Hospital in Singapore to develop the technology further for use in real clinical settings.
More information: Jiahong Chen et al, Rapid and Accurate Antimicrobial Susceptibility Testing Using Label‐Free Electrical Impedance‐Based Microfluidic Platform, Small (2023). DOI: 10.1002/smll.202303352
Journal information: Small
Provided by Singapore University of Technology and Design