This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Optimizing the Tet-On system for homogeneous iPSC myogenic differentiation
In a recent study published in iScience, researchers have optimized the Tet-On system to improve the efficiency of generating skeletal muscle cells and other differentiated cell types from iPSCs for various basic and clinical research purposes. The team was led by Associate Professor Hidetoshi Sakurai (Department of Clinical Application) and Associate Professor Knut Woltjen (Department of Life Science Frontiers).
A crucial challenge for researchers in producing differentiated cells and tissues from induced pluripotent stem cells (iPSCs) is heterogeneity. For example, contamination by undifferentiated or partially differentiated cells causes reproducibility and safety issues for disease modeling and cell therapy, respectively.
The tetracycline-inducible gene expression system (Tet-On) is a fundamental genetic tool employed by researchers to regulate transgene expression in response to doxycycline, a common antibiotic. Specifically, the system is based on the ability of the reverse tetracycline transactivator (rtTA) to bind the Tet-On promoter, consisting of Tet-operon repeats and the minimal cytomegalovirus (CMV) promoter, only in the presence of a drug called doxycycline to activate exogenous gene expression.
Its ease of use allows researchers to initiate direct differentiation of iPSCs into various cell types. However, non-uniform transgene activation remains problematic, and therefore researchers must evaluate and select iPSC clones to identify ones that express the transgene of interest in an efficient and stable manner.
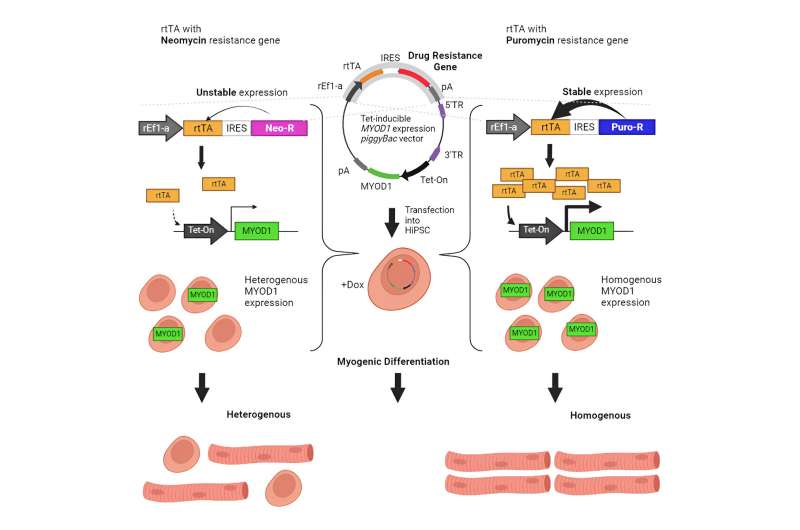
To tackle the heterogeneity problem, the researchers first returned to the basics to identify the root cause of failed transgene activation. Since MYOD1 overexpression is the preferred induction method to drive myogenic differentiation from iPSCs, the researchers first examined the ability of a piggyBac transposase expression system to overexpress MYOD1 (with mCherry as a fluorescent reporter protein to indicate MYOD1 expression in parallel), rtTA, and neomycin (antibiotic)-resistance positive selection marker.
After introducing the piggyBac system into iPSCs and selecting cells with the system integrated into their genome, cells were treated with doxycycline to initiate myogenic differentiation. Notably, the researchers found that not all cells expressed MYOD1, suggesting many cells failed to turn the transgene on.
To determine the cause, they separated cells with and without mCherry, based on the fluorescence signal they emitted, for further analysis. Interestingly, although they found non-fluorescent cells to contain the exogenous gene, rtTA expression was much lower when compared to fluorescent cells.
Based on this observation, the researchers tested whether supplementing rtTA expression would enhance the efficiency of myogenic differentiation. As they had hypothesized, rtTA supplementation increased differentiation efficiency from about 40% to more than 80%, thus indicating that rtTA expression may be a bottleneck for iPSC differentiation.
The researchers next examined another factor that may influence transgene expression: The antibiotic resistance positive selection marker. To test this parameter, they replaced the neomycin resistance transgene with one that encodes for puromycin resistance in the piggyBac system. Surprisingly, even though the cells had fewer copies of the transgene with puromycin resistance, not only did they express high levels of mCherry and rtTA, but the relative proportion of cells expressing the fluorescent reporter protein was significantly increased simply by switching antibiotic resistance.
The researchers then tested whether this switch would promote uniform MYOD1 expression and myogenic differentiation. Indeed, they reproducibly achieved more than 90% differentiation efficiency using the puromycin resistance selection instead.
By optimizing the described factors and other parameters, the research team showed that they could successfully differentiate iPSCs into skeletal muscle cells at a sufficient efficiency that clone evaluation and selection were unnecessary. Equipped with this new knowledge, researchers can adopt similar strategies for other differentiation methods required to generate various cell types for reproducible disease modeling and safe cell therapy.
More information: Jun Otomo et al, Uniform transgene activation in Tet-On systems depends on sustained rtTA expression, iScience (2023). DOI: 10.1016/j.isci.2023.107685
Journal information: iScience
Provided by Kyoto University