This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
DNA aptamer finds novel application in regulating cell differentiation
Generating specific cell lineages from induced pluripotent stem cells and embryonic stem cells is the holy grail of regenerative medicine. Guiding iPSCs toward a target cell line has garnered much attention, but the process remains challenging.
Now, researchers from Japan have discovered that an anti-nucleolin DNA aptamer, iSN04, can determine a cell's lineage during differentiation. By demonstrating the generation of cardiomyocytes from murine pluripotent stem cells, their concept shows promise as a regenerative therapy.
Self-renewal and pluripotency–the capacity to form any cell lineage–are inherent characteristics of induced pluripotent stem cells (iPSCs). Furthermore, they are highly prized in regenerative therapies targeting cardiovascular, neurological, and metabolic diseases as they are immunologically suitable for transplantation back into a donor.
Unfortunately, regenerative medicine is not yet feasible outside a laboratory setting as available protocols to generate target cells are complicated and expensive. This raises a pertinent question: Can regulating the fate of stem cells in clinical settings and at scale be made more economical?
A team of researchers from Shinshu University, the National Institute of Advanced Industrial Science and Technology, and the University of Shizuoka in Japan set out to address this question by leveraging nucleic acid aptamers.
Aptamers are single-stranded pieces of DNA that bind to target proteins and are able to modulate signaling cascades during cell differentiation when a stem cell commits to a specific functional role or phenotype. They hold promise in regenerative medicine as they are easily modified, can be synthesized economically, and are suitable for long-term storage.
The team, led by Associate Professor Tomohide Takaya from the Department of Agricultural and Life Sciences at Shinshu University, recently discovered that an anti-nucleolin aptamer, myogenetic oligodeoxynucleotide iSN04, induced myocardial differentiation in embryonic stem cells (ESCs). The study was led by Mina Ishioka, a graduate student in Dr. Takaya's laboratory, and published in The International Journal of Molecular Sciences.
"We had previously found that iSN04 promoted myogenic precursor cells (myoblasts) to differentiate into skeletal muscle cells and had hypothesized that the aptamer also enhanced differentiation of pluripotent stem cells. We were intrigued by the prospect of using iSN04 to promote iPSC differentiation into cardiomyocytes as this could lead to regenerating heart tissue," says Dr. Takaya, elaborating on the team's motivation to pursue the research.
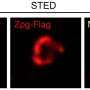
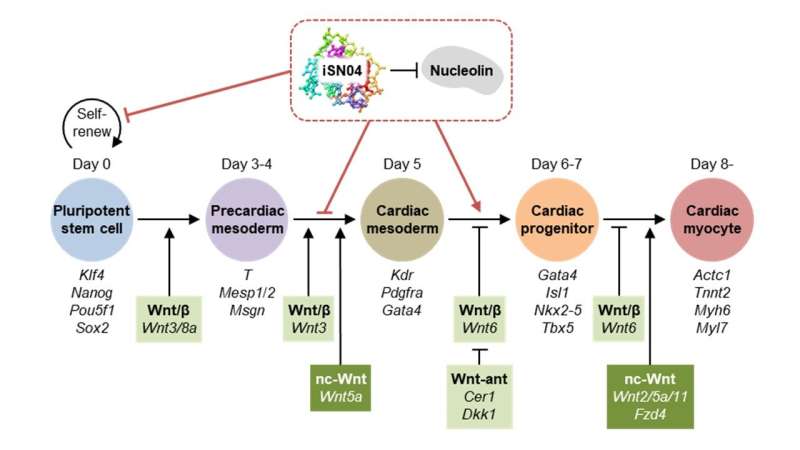
Using various assays like RNA sequencing, cell staining and imaging, and molecular interaction and pathway analysis, the researchers investigated iSN04's effect on murine ESCs and iPSCs. iSN04 treatment under differentiating conditions inhibited stem cell commitment to the cardiac lineage. However, when these pluripotent stem cells were treated after experiencing differentiating conditions for five days, specific marker genes were upregulated, and the cells committed to forming beating cardiomyocytes.
"Ours is the first report to confirm a DNA aptamer that allows cardiomyocytes to develop from iPSCs," explains Dr. Takaya when asked about the significance of the work.
"We uncovered two mechanisms of nucleolin interference with iSN04 at play whereby early treatment inhibits cardiomyogenesis, while treatment at a later stage enhances the generation of cardiac progenitors. First, iSN04 governs the translocation of nucleolin protein between the cytoplasm, plasma membrane, and nucleus. Second, it results in the modulation of the Wnt signaling pathway that governs cell differentiation."
The immunostaining experiments revealed that nucleolin was retained in the nucleoli following iSN04 treatment. Nucleolar nucleolin has a role in chromatin remodeling and gene transcription, and interestingly enough, Wnt pathway genes were differentially expressed in the RNA-seq data following iSN04 suppression. The team postulates that the iSN04-anchored nucleolin alters gene expression and Wnt signaling. Ultimately, terminal cell differentiation commits to the cardiomyocyte lineage.
And how could these findings impact regenerative medicine and patients' lives in the long term? Dr. Takaya provides insights into the broader implications of their work.
"We believe there is a strong case to be made for further studies evaluating DNA aptamers in regenerative medicine. Aptamers are cost-effective and open up the possibility of producing specific cells from the patient's stem cells. But it doesn't end there. Since the aptamers can regulate stem cell fate, they can serve as therapeutic agents for many conditions linked to stem cell dysfunction," he concludes.
More information: Mina Ishioka et al, Myogenetic Oligodeoxynucleotide Induces Myocardial Differentiation of Murine Pluripotent Stem Cells, International Journal of Molecular Sciences (2023). DOI: 10.3390/ijms241814380
Journal information: International Journal of Molecular Sciences
Provided by Shinshu University