This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Material cycle for amine chemistry: Important building blocks created from platform chemical in single step
Fossil raw materials still dominate the chemical industry. But laboratories around the world are researching ways in which large-scale processes can avoid crude oil, natural gas and coal in the future. So-called platform chemicals are gaining in importance; they are produced entirely from renewable raw materials. But their use in industrial processes requires special catalysts.
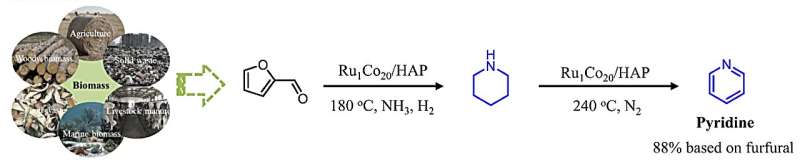
A team of researchers at LIKAT, in cooperation with the Dalian Institute of Chemical Physics, has just presented such a catalyst in the journal Nature Communications. In a single reaction step, it allows the platform chemical furfural to be converted into amines, which are among the most important synthesis building blocks.
Amines are functional components in the synthesis of drugs and agrochemicals, and they are used en masse in many fields, such as energy technology and materials science. Ultimately, they introduce into chemical processes those nitrogen units that provide specific properties. The amine market is growing strongly, with estimates predicting annual growth rates of 8% over the next ten years.
Reaction without waste and by-products
This growth attracted Haifeng Qi from Dalian to take a closer look at this reaction in his dissertation. "Because amine synthesis is still largely dependent on fossil resources," he says. At the same time, interest is growing worldwide in cost-effective methods for their sustainable production based on renewable raw materials, explains Dr. Kathrin Junge, in whose research group Dr. Qi is currently working as a Humboldt Fellow at LIKAT, the Leibniz Institute for Catalysis in Rostock.
The new reaction proceeds in a single step, instead of the previous five to six steps. Qi uses the platform chemical furfural, which is produced entirely from biowaste, as well as ammonia and hydrogen. This produces the amine piperidine, an intermediate for pharmaceuticals, crop protection agents and solvents, among other things.
As a model reaction, this process can be used universally. "For example, if you further heat the product piperidine and at the same time turn off the supply of hydrogen and ammonia, another amine called pyridine is formed," Dr. Qi reports of his research. Both times he completely converted his starting materials, no waste products were formed.
Yield of almost 100%
This also means that the usual purification of the amines can be dispensed with after these processes, as Dr. Junge says. "The catalyst can also be easily reused for new cycles." Just how highly selective it is in controlling the reaction is shown by the yield, which is up to 97%. Basically, a sensational value.
How does the catalyst manage that? Dr. Qi smiles when he hears the question, because that's exactly what he also asked himself when he recognized its highly selective approach. That's why the catalyst was precisely examined in the analysis area of LIKAT.
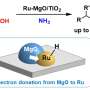
Qi had prepared his catalyst from cobalt and ruthenium in the usual way for heterogeneous catalysis: He dissolved salts of the two metals in water, added a support material to which the metal could settle, then allowed the solvent to evaporate and the complex to dry. He then exposed his catalyst to a heat of 400°C. Chemists call this process pyrolysis: The material does not burn, but changes its structure.
Individual atoms cause the effect
How decisively the heat had changed the catalyst structure was later revealed under the high-tech microscopes. Dr. Junge explains, "Groups of cobalt atoms came together in nanoparticles, on whose surface the ruthenium was deposited, in the form of individual atoms."
Qi states, "It was exactly this single-atom structure, as we call it, that made the effect. And it's quite stable." All of this makes for a fairly simple arrangement for this amine production that Dr. Junge believes any lab technician could handle.
"Such a material cycle of amine production based on biomass is hardly known," emphasizes LIKAT Director Prof. Dr. Matthias Beller, who supervised Qi's work from the German side. This could be the basis of a "biorefinery of the future."
More information: Haifeng Qi et al, Synthesis of piperidines and pyridine from furfural over a surface single-atom alloy Ru1CoNP catalyst, Nature Communications (2023). DOI: 10.1038/s41467-023-42043-6
Journal information: Nature Communications
Provided by Leibniz Institute for Catalysis