October 17, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Direct imaging of sequences and locations of glycans bound to biomolecules at a single-molecule level
A team of organic chemists at the Max-Planck Institute for Solid-State Research, working with colleagues from the University of Tübingen and the University of Copenhagen, reports a way to take pictures of the sequences and locations of glycans (also known as polysaccharides) bound to several biomolecules at the single-molecule level. Their study is published in Science.
Glycans are types of carbohydrates involved in a myriad of biological processes, one of which is protein folding. They are typically found on the exteriors of most cells. Prior research has shown that they can take either a branched or linear form and are made of O-glycosidic linkages of monosaccharides. Because of their importance in both biological processes and research efforts, scientists have been studying them for many years. In this new effort, the research team developed a microscopy method to take pictures of glycans as they bind to proteins.
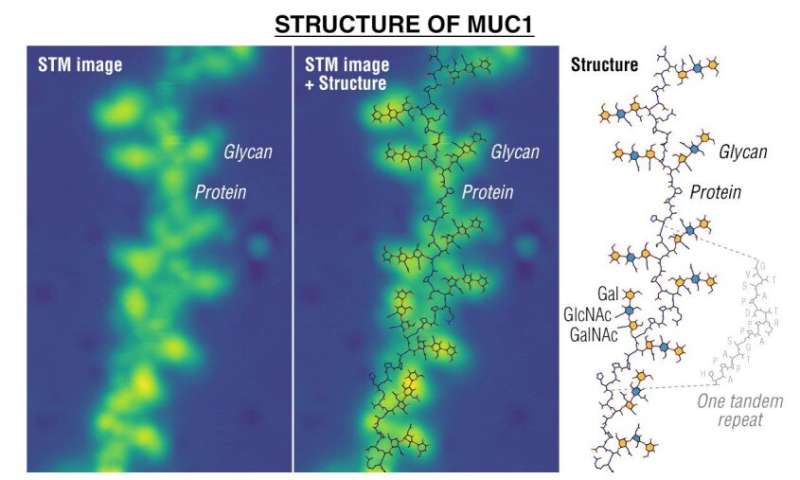
After conducting a multitude of experiments looking for a way to image glycan binding, the team found one that involved an electrospray technique that pushed glycans bound to lipid and protein molecules (known as glycosaminoglycans and glycoconjugates) onto two metal surfaces—silver and copper. This allowed them to image the molecules directly using scanning tunneling microscopy.
The researchers were able to identify given monosaccharides in a glycan chain, which in turn allowed them to learn more about how the glycans are oriented and their attachment position on protein backbones.
The researchers also demonstrated their new imaging technique by creating pictures of oxygen-linked glycans as they were bound to mucin proteins—images, they note, that could be useful in the search for early cancer biomarkers. The technique could be used in a wide variety of research efforts, perhaps even in helping to find unknown glycolipids and/or glycoproteins.
More information: Kelvin Anggara et al, Direct observation of glycans bonded to proteins and lipids at the single-molecule level, Science (2023). DOI: 10.1126/science.adh3856
Journal information: Science
© 2023 Science X Network