This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Gene integral to initiating, sustaining sperm cell development identified

With male infertility a mounting global concern impacting approximately 12% of men, according to the National Institutes of Health, a Penn State research team has discovered a gene that plays a key role in initiating and sustaining spermatogenesis. The finding, they said, may open a door for future therapies to boost sperm counts.
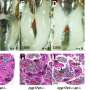
In a recent study published in Development, the researchers reported that the gene PRAMEL1 is critical to the initiation and maintenance of spermatogenesis, the process of producing motile—capable of motion—spermatozoa. Spermatozoa, typically having a compact head and a long, threadlike tail for swimming, are the mature male sex cell of an animal by which the female egg is fertilized.
"By analyzing mouse models with PRAMEL1 inactivated, we discovered that the gene finely regulates genetic signaling mechanisms, playing a pivotal role in the proper establishment of both the initial and subsequent rounds of spermatogenesis," said team leader Wansheng Liu, Penn State professor of animal genomics.
Over the past four decades, sperm count in men has plummeted by 52%, according to Liu. Moreover, this decline persists at an annual rate of 1.4% in Western populations, and conditions like azoospermia—absence of viable sperm—and oligozoospermia—low sperm levels—are increasingly prevalent.
This decline in sperm production constitutes a substantial societal and biological challenge, Liu pointed out.
"Unfortunately, our understanding of spermatogenesis remains limited, making it challenging to devise strategies to mitigate and address infertility," he said. "Our recent work focuses on the molecular and cellular mechanisms underlying the initiation and maintenance of spermatogenesis in mammals."
The researchers focus their work on PRAME, a multicopy gene family that comprises approximately 60 members in the human genome and around 90 members in the mouse genome. PRAME proteins are predominantly expressed in gonadal tissues—testis and ovary.
For a considerable period, scientists encountered significant challenges in deciphering the functional roles of the genes in the PRAME family in reproduction due to the extensive family size and overlapping expression patterns, Liu explained. But in previous studies conducted in his lab in the College of Agricultural Sciences—led by Mingyao Yang, who recently completed her doctoral training in Liu's lab—the researchers began to comprehend the role of PRAME genes.

"That's when we decided to see what would happen if we deleted these genes, to learn what's the effect of this protein during spermatogenesis," said Yang, who is the first author on the paper reporting on the role of PRAMEL1. "In our recent research breakthrough, we successfully knocked out two members within the mouse PRAME family, uncovering novel insights into the roles of the PRAME family in initiating and sustaining spermatogenesis."
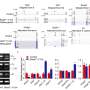
Collectively, these findings strongly indicate that PRAMEL1 finely regulates retinoic acid signaling, she said. Retinoic acid is essential for the commitment of sperm cells to spermatogenesis in the testis, so this signaling plays a pivotal role in the proper establishment of both the initial and subsequent phases of spermatogenesis
However, the functioning of PRAME genes in spermatogenesis is complex, noted Yang, who recently successfully defended her doctoral thesis on the mouse PRAME research. Both she and Liu were surprised to find that PRAMEL1 deficiency led to increased fecundity—the ability to produce an abundance of offspring—in juvenile males, while PRAMEL1 deficiency had the opposite effect in mature males, causing decreased fecundity.
"So, you can see the same gene family could have a complicated function in age-related reactions," Liu said.
The fact that the expression of PRAME genes affect young and older mice differently may have implications for the human male infertility crisis, Liu suggested.
"That was supposed to be a consistent function, right?" Liu asked. "We didn't expect to find that the function of these genes in young animals versus older animals is different. We discovered a very tricky part—something much more complicated than we were thinking about gene regulation. It will take more research to understand it."
More information: Mingyao Yang et al, The mouse Pramel1 regulates spermatogonial development by inhibiting the retinoic acid (RA) signaling during spermatogenesis, Development (2023). DOI: 10.1242/dev.201907
Journal information: Development
Provided by Pennsylvania State University