Novel role of progestin signaling in fish spermatogenesis
Cytochrome P450, family 17, subfamily A, polypeptide 1 (cyp17a1) is a critical enzyme involved in gonadal steroidogenesis, including androgen and estrogen production. Among sex steroids, androgen signaling disruption is known to cause testicular malformation and defective spermatogenesis in zebrafish. Interestingly, enhanced spermatogenesis has been observed in cyp17a1-deficient zebrafish and common carp, with the impaired process of the androgen production.
On the other hand, even though progestin signaling has been suggested to play important roles in spermatogenesis in certain animals, when the proper progestin signaling had been abolished in nuclear progesterone receptor (npgr)-deficient zebrafish, no defective spermatogenesis has been seen previously.
In a study published in eLife, a research team led by Prof. Yin Zhan from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences provides compelling evidence demonstrating that an augmentation of progestin/nPgr signaling can sufficiently compensate for proper spermatogenesis and testis organization when androgen signaling is depleted in zebrafish.
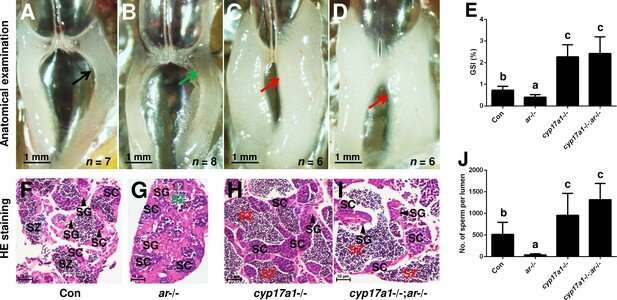
For the aspect of androgen signals, its active signaling should be similarly disrupted in both androgen receptor (ar)- or cyp17a1-deficient zebrafish. However, impaired spermatogenesis has been seen in ar-deficient zebrafish, but not in cyp17a1-deficient fish. When the cyp17a1-/-;ar-/- fish were generated, enhanced spermatogenesis has been achieved in this double knockout model. These results suggested that cyp17a1 knockout activates a compensatory pathway that promote testis organization and spermatogenesis independent of Ar signaling.
The researchers subsequently measured the whole-body contents of 11-ketotestosterone (11-KT), 17α, 20β-dihydroxy-4-pregnen-3-one (DHP), and progesterone (P4) of the control males and cyp17a1-/- fish at 3, 3.5, and 4 mpf using ultra performance liquid chromatography tandem mass spectrometry. A significant increased levels of DHP and P4 in the cyp17a1-/- fish, but in ar-deficient fish, were observed.
To confirm possible roles of the increased concentration of DHP responsible for testis organization and spermatogenesis in the zebrafish with the deficiency of the androgen signaling, the researchers treated the ar-/- males with 0.067 μg/mL DHP from 50 to 90 days post-fertilization. The effective restoration of testis organization and spermatogenesis after DHP treatment in ar-/- males confirmed that the augmentation of progestin signals might facilitate testicular development and spermatogenesis by compensating for the deficiency of androgen signaling in the cyp17a1-/- fish.
To test the potential roles of the high progestin signaling in the fish disruption of androgen signaling, the npgr mutation was then introduced into the cyp17a1-/-;ar-/- fish. Compared with those in the cyp17a1-/- and cyp17a1-/-;ar-/- fish, greater spermatogonia and spermatocytes, and less spermatozoa in the ar-/- males, ar-/-;npgr-/- males, and cyp17a1-/-;ar-/-;npgr-/- fish were observed in the histological analyses of testes. These results suggested that the compensatory function of the progestin signaling augmentation induced by cyp17a1-depletion is nPgr-dependent.
Furthermore, the researchers performed comparative analyses of transcriptomes in the testes of the control males, ar-/- males, cyp17a1-/- fish, npgr-/- males, cyp17a1-/-;ar-/- fish, cyp17a1-/-;npgr-/- fish, ar-/-;npgr-/- males, and cyp17a1-/-;ar-/-;npgr-/- fish. They found that insulin-like 3 (insl3) may be a target that is co-regulated by androgen signaling and high level of progestin signaling.
This study uncovers the role of augmentation of progestin signals in promoting testis organization and spermatogenesis, which is alternative and independent of androgen signaling (in cyp17a1-/- fish and cyp17a1-/-;ar-/- males). This sheds light on the functional understanding of ancestral progestin signaling during steroidogenesis evolution.
More information: Gang Zhai et al, Augmentation of progestin signaling rescues testis organization and spermatogenesis in zebrafish with the depletion of androgen signaling, eLife (2022). DOI: 10.7554/eLife.66118
Journal information: eLife
Provided by Chinese Academy of Sciences