This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New ammonia reaction could offer a sustainable source of nitrogen
A big goal in chemistry is to find a simple way to produce amines from ammonia and unsaturated hydrocarbons. Catalytic addition to activate and transfer ammonia would not give rise to any waste. Hence, the process would be sustainable.
Researchers from Karlsruhe Institute of Technology (KIT) have now come closer to reaching this goal. They have developed a system for activation and catalytic transfer of ammonia, which is not based on transition metals, but on a compound of main group elements. The results are reported in Nature Chemistry.
The ammonia molecule (NH3), a compound of nitrogen and hydrogen, is one of the most frequently produced chemicals worldwide and it is also used for the production of many other nitrogen-containing compounds.
If amines could be produced by the simple addition of ammonia to unsaturated hydrocarbons, this would be a major breakthrough in chemistry, because amines, the organic derivatives of ammonia, are in high demand as building blocks of agricultural and pharmaceutical chemicals as well as of detergent substances, dyes, lubricants, and coatings. Moreover, amines are used as catalysts in the production of polyurethanes. And amines are applied in gas scrubbers at refineries and power plants.
By breaking the strong bond between nitrogen and hydrogen, i.e., activation, the ammonia molecule may be transferred at least theoretically to other molecules, such as unsaturated hydrocarbons. Transfer of ammonia to ethylene, an important substance in chemical industry, for instance, would give rise to ethylamine. This addition is referred to as hydroamination by chemists. However, ammonia and ethylene do not react with each other easily. A catalyst is required for the reaction to take place.
Conventional catalysts based on transition metals, however, react with ammonia and become inactive. "Hydroamination of non-activated alkenes with ammonia, hence, is considered a big challenge or the holy grail of catalysis," says Professor Frank Breher, head of a research group at the Division of Molecular Chemistry of KIT's Institute for Inorganic Chemistry (AOC).
Activation and catalytic transfer of ammonia
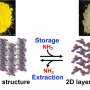
In cooperation with researchers from Paderborn University and Complutense University of Madrid, Professor Frank Breher and Dr. Felix Krämer from AOC have now come much closer to reaching this challenging goal. "We have developed a system for the activation of ammonia, which is not based on transition metals, but on main group elements. The 'atom-economic' process of activation and subsequent transfer of ammonia do not give rise to any waste, which is of particular interest in terms of sustainability," Breher says.
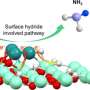
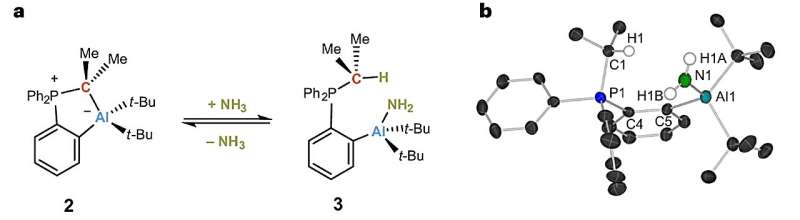
The team produced a so-called frustrated Lewis pair (FLP) that consists of an acid as electron pair acceptor and a base as electron pair donor. Usually, both react with each other and produce an adduct. If adduct formation is prevented or at least limited, a frustrated situation results and the molecule readily reacts with small molecules, such as ammonia.
"It is crucial to dampen reactivity such that the reaction with small molecules is reversible. Only then will it be possible to use such an FLP in catalysis. We were the first to achieve this with ammonia as a substrate," Breher reports.
The FLP was found to easily react with non-aqueous ammonia in a thermoneutral way and to reversibly split the nitrogen-hydrogen bond of ammonia at room temperature. For the first time, the researchers present NH3 transfer reactions catalyzed by a catalyst based on main group elements.
"So far, we have converted activated substrates only and no unsaturated hydrocarbons. But we have come much closer to the reaction of our dreams," Breher says. "We expect that our first proof of principle will initiate further work on the use of N-H-activated ammonia as an easily available and sustainable source of nitrogen."
More information: Felix Krämer et al, A crystalline aluminium–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01340-9
Journal information: Nature Chemistry
Provided by Karlsruhe Institute of Technology