This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
How do zeolite-encapsulated metal catalysts act on hydrogen-related catalytic reactions?
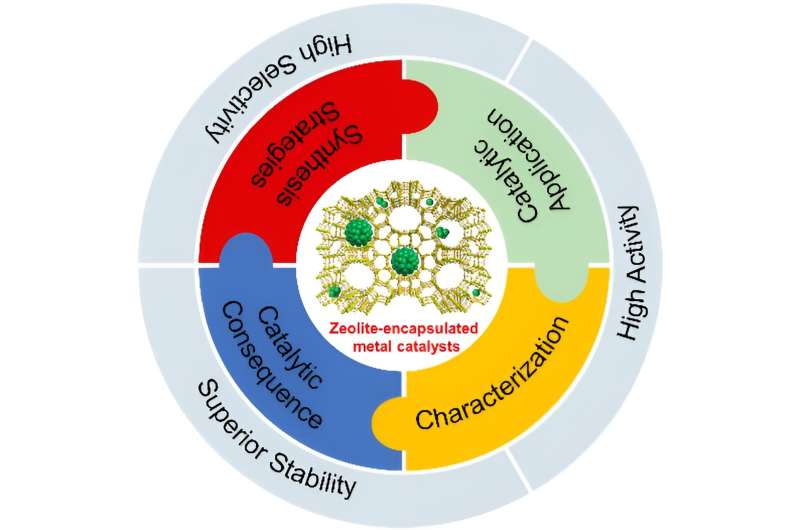
Zeolites encapsulated metal and metal oxide species (regarded as metal@zeolite) are an important type of heterogeneous catalyst. They give performances that steadily outperform the traditional supported catalysts in many important reactions and have become a research hotspot. Remarkable achievements have been made dealing with the synthesis, characterization, and performances of metal species (typically metal and metal oxide clusters) confined in zeolites.
Although tremendous progress has been made with the synthesis, characterization, and catalysis of metal@zeolite catalyst, there remain many challenges that needed to be addressed. A team of scientists summarized the progress of metal@zeolite catalyst in recent years. Their work was published in Industrial Chemistry & Materials.
Metal catalysts are an important class of catalytic materials, which have been widely used in various chemical reactions such as redox, hydrogenation, and coupling reactions in the past few decades. It has been extensively reported in the literature that the small metal particle size contributes to greater exposure of active sites, as well as higher reactivity.
"However, small metal particles generally have low thermal stability and tend to sinter, coalesce, or leach under harsh reaction conditions, even when they have a strong interaction with supports. Therefore, deactivation due to sintering and leaching during the reaction process is one of the most important problems for the practical application of metal catalysts with small sizes," said Zhijie Wu, a professor at the China University of Petroleum-Beijing.
Currently, confining metal species within graphene oxide, nanoporous carbon, metal-organic frameworks, and zeolite is a promising method to solve this problem.
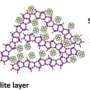
Among them, zeolite has a high specific surface area, high thermal and hydrothermal stability, and adjustable acid-base properties, which has become an ideal support for confining highly dispersed metal species. In recent years, zeolites-encapsulated metal catalysts have been widely used in various catalytic processes, and their catalytic performance is steadily better than that of traditional supported catalysts, which has become a research hotspot.
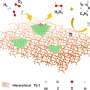
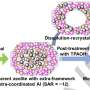
Based on the origin of metal species within zeolites, three types of zeolite-encapsulated metal catalysts can be assigned. First, metal atoms embedded within the zeolite framework are removed and transferred into zeolite channels or pores via the calcination or reduction process.
Second, metal species without the capability to incorporate into a zeolite framework (e.g. Pt, Pd, or Ni) are introduced within zeolite micropores and then calcinated or reduced to encapsulated metal or metal oxides. Third, metal particles within the intra-crystalline mesopore of zeolites.
Two typical synthesis strategies for zeolite encapsulation, including post-treatment (i.e., ion-exchanging, interzeolite transformation, recrystallization, etc.) and in-situ synthesis (i.e., in-situ hydrothermal, dry-gel synthesis, etc.), have been developed. Specially, the in-situ hydrothermal synthesis and dry-gel conversion are frequently developed.
Because of the confinement effect within zeolite micropores, zeolite encapsulated metal or metal oxides show small size, even in sub-nanometer or atomic scale. Moreover, the geometric and electronic properties of encapsulated metal species are complex because of the strong metal-framework interaction.
Therefore, identifying metal particles confined in zeolites is challenging. Common characterization tools including X-ray diffraction, X-ray photoelectron spectroscopy, and transmission electron microscopy have been used to demonstrate whether the metal species are encapsulated in the micropores of zeolites.
However, these characterizations cannot reveal the fine structure of the metal species as well as the coordination environment in zeolite-encapsulated metal catalysts. Therefore, advanced characterization techniques such as spherical aberration-corrected scanning transmission electron microscopy (Cs-corrected STEM), X-ray absorption spectroscopy (XAS), and low-temperature CO infrared spectroscopy (CO-FTIR) have been developed to enable the study of the fine structure of the metal sites at the atomic level.
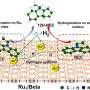
Encapsulation of metal species within zeolites is an attractive route to contain and maintain small and uniform metal clusters, to protect them against sintering and poisoning, and to select reactants, products, and transition states in catalytic reactions. However, the confinement of metal species within zeolites may also increase the diffusion barrier of reactant or product throughout the zeolite aperture, leading to a decreasing reaction rate somewhat.
Again, the confinement inevitably sacrifices some acid sites via the interaction between metal species with the zeolite framework. These together make the catalytic consequence of the zeolite-encapsulated metal catalysts complex which is varied with the reaction conditions.
"In the past decade, zeolite-encapsulated metal catalysts have been used in many catalytic processes and exhibit excellent performance. In this review, applications of metal@zeolite catalyst in hydrogen-related reactions were summarized," said Wu.
There is still huge room for metal@zeolite in future development. Non-noble metals are more prone to agglomerate into large particles under the synthesis and reduction process. So far, zeolite-encapsulated ultra-small non-noble metal catalysts, especially those with high thermal stability, are still rarely reported.
Thus, effective methods to confine ultra-small non-noble metal clusters or single non-noble metal atom in zeolites is significant for developing efficient non-noble metal catalyst. In addition, the chemical states and coordination environment of metal species confined in zeolites may change in the reaction progress. In further research, more effects should be devoted to the development of in situ or operando characterization techniques and the design and optimization of the synergic effect between metal species and acid functions.
"Through this review, researchers can further understand the confinement effect of zeolites. At the same time, it provides a reference for designing zeolite-encapsulated metal catalysts with better performance," said Wu
More information: Meng Liu et al, Recent advances in the synthesis, characterization, and catalytic consequence of metal species confined within zeolite for hydrogen-related reactions, Industrial Chemistry & Materials (2023). DOI: 10.1039/D3IM00074E
Provided by Industrial Chemistry & Materials