This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Team resolves molecular switching behavior of azonium compounds for light-controlled drugs
Molecules that change shape under the influence of light can be used as switches in biomedical applications, for instance to inhibit an enzyme. An international team of researchers, including chemists at the Universities of Amsterdam and Groningen, have now resolved the fundamentals of the molecular switching behavior of a specific class of switchable molecules called azonium compounds.
In the Journal of the American Chemical Society, they present a quantitative analysis based on advanced laser spectroscopy, quantum chemical modeling and theoretical calculations. It paves the way towards actual application of these compounds in developing light-controlled drugs.
Molecules capable of shape-shifting under the influence of light are studied by many chemists worldwide, as they allow precise control over molecular processes. As an example, light-controlled molecular motors earned Professor Ben Feringa his 2016 Nobel Prize in Chemistry.
A particularly relevant field of application is that of photopharmacology, where light-switchable molecules are used to control physiological processes. Light-induced molecular shape-shifting can, for instance, induce the blocking of an ion channel or inhibition of an enzyme. Using light to precisely control such therapeutical processes at the right time and at the right place can limit unwanted side effects.
Professor Wiktor Szymanski, a colleague of Feringa at the University of Groningen, is working toward such applications. As a professor of photopharmacology, he has a special interest in a particular type of molecules called azonium compounds that were first developed by Prof. Andrew Woolley at the University of Toronto (Canada).
These compounds constitute one of the very few molecular systems that tick all the boxes for use in photopharmacology. They can be switched using red or infrared light, which is safe for humans and can penetrate deep into living tissue. They are also stable under the conditions of found in most parts of the human body—an aqueous environment at pH 7. Furthermore, their shape shift is quite persistent, at least long enough to trigger a biological response. And finally, the molecules retain their functionality after multiple switching events.
First detailed mechanistic insight
To effectively use these molecular switches for photopharmaceutical applications requires a thorough knowledge of their switching behavior. This was investigated by a multidisciplinary, international team where synthetic chemists Szymanski and Feringa worked together with spectroscopists Prof. Wybren Jan Buma (University of Amsterdam) and Mariangela di Donato (LENS research center, Florence), and computational chemists Miroslav Medved' (Matej Bel University, Slovak Republic) and Adèle Laurent (Nantes University). Having already successfully collaborated on the development of novel photoswitches, they now also worked with azonium switches pioneer Andrew Woolley, who coordinated the research.
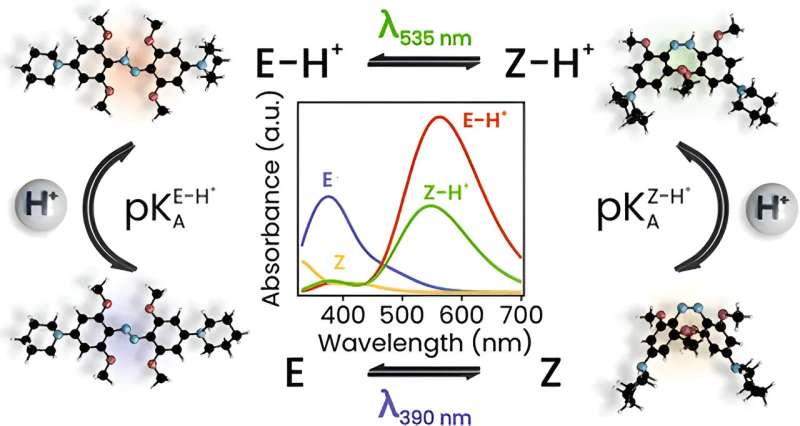
In JACS they present the first detailed mechanistic insight into the photochemistry of azonium ions. It was obtained using a combination of time-resolved spectroscopies (on time scales ranging from picosecond to seconds), quantum chemical calculations, and theoretical analysis. The results reveal how photon absorption leads to a change in molecular conformation, how the exchange of a proton with the solvent stabilizes this shape-shift, and how the pH of the solution governs the relaxation rate of the switch after a light pulse.
The analysis provides the first complete mechanistic picture that explains the observed intricate photoswitching behavior of azonium ions at a range of pH values. The research also leads the way to the modification of azonium ions to enhance their applicability for the photocontrol of biomolecules. The first steps have already been taken towards implementation of these photoswitches in molecular systems that interact with living cells. Although still in its infancy, it constitutes a promising development towards a future generation of smart medicines.
More information: Miroslav Medved' et al, Mechanistic Basis for Red Light Switching of Azonium Ions, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c06157
Journal information: Journal of the American Chemical Society
Provided by University of Amsterdam