This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers report photoclick reactions with unprecedented efficiency
In a cooperative effort between the Universities of Groningen and Amsterdam (The Netherlands) and the European Laboratory for Non-Linear Spectroscopy (Italy), researchers have been able to substantially improve photoclick chemistry. The team was able to boost the reactivity of the photoclick compound in the popular PQ-ERA reaction through strategic molecular substitution.
In their article published in the journal Chemical Science, the researchers report a superb photoreaction quantum yield, high reaction rates and notable oxygen tolerance. The paper was designated a HOT Article as well as Pick of the Week.
At the University of Amsterdam, Michiel Hilbers and Wybren Jan Buma of the Molecular Photonics group (Van 't Hoff Institute for Molecular Sciences) contributed to the research. The synthetic and reaction characterization was conducted in the laboratories of Wiktor Szymanski and Nobel laureate Ben Feringa at the University of Groningen.
Photoclick chemistry is a light-activated variety of click chemistry (Nobel Prize in Chemistry 2022), a set of elegant and efficient chemical reaction methods that couple dedicated molecular units to yield desired products. Photoclick chemistry has unique advantages over conventional click chemistry as it allows for a high degree of both spatial and temporal control over the reaction. It has a broad range of applications, including 3D printing, protein labeling, and bioimaging.
A boost for the PQ-ERA photoclick reaction
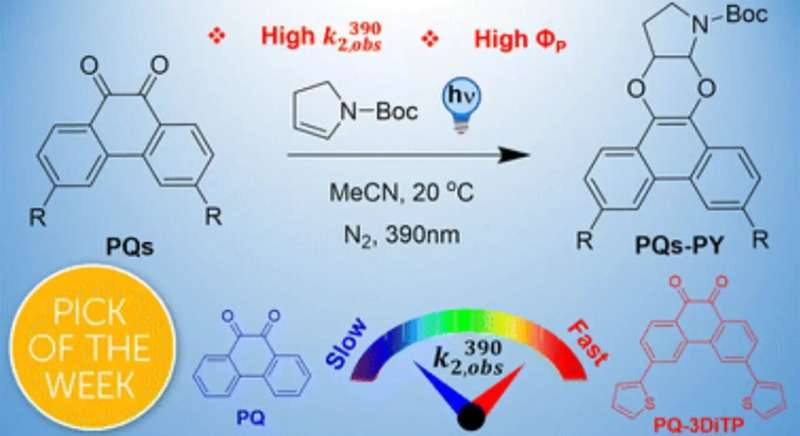
A specific photoclick reaction is the so-called PQ-ERA reaction—the light-induced photocycloaddition of 9,10-phenanthrenequinone (PQ) with electron-rich alkenes (ERA). It has drawn much attention because of its excellent kinetics and biocompatibility. However, the conventionally used PQ compounds show limited reactivity, which hinders its overall efficiency.
In the study now presented in Chemical Science, the international research team offers a simple strategy to change that. They describe how a thiophene substitution at the 3-position of the PQ scaffold significantly boosts the reactivity of the PQ triplet state to enhance the efficiency of the PQ-ERA reaction. Nanosecond time-resolved spectroscopic studies and quantum chemical studies in the Amsterdam Molecular Photonics group combined with femtosecond time-resolved spectroscopic studies performed in Florence provided a fundamental understanding of this specific photoclick chemistry.
The investigations show that the substitution significantly increases the population of the reactive triplet state (3ππ*) during excitation of 3-thiophene PQs. This results in a superb photoreaction quantum yield (FP, up to 98%), high second order rate constants (k2, up to 1974 M−1 s−1), and notable oxygen tolerance for the PQ-ERA reaction system.
These results now pave the way for a further improvement of the reaction, offering excellent prospects for fast and efficient photoclick transformations.
More information: Youxin Fu et al, Establishing PQ-ERA photoclick reactions with unprecedented efficiency by engineering of the nature of the phenanthraquinone triplet state, Chemical Science (2023). DOI: 10.1039/D3SC01760E
Journal information: Chemical Science
Provided by University of Amsterdam