This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Solid-state NMR unveils fluoride ion channel permeation mechanism
A research team led by Shi Chaowei from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) has adapted the fluoride ion channel protein Fluc-Ec1 combined with deuterium substitution and 19F labeling methods, paving a new path for membrane protein nuclear magnetic resonance (NMR) research.
The researchers published a paper titled "Fluoride permeation mechanism of the Fluc channel in liposomes revealed by solid-state NMR" in Science Advances.
NMR not only provides insights into molecular structures but also observes their dynamic characteristics. These insights are invaluable for understanding the functional mechanisms of large biomolecules such as proteins. With the advancement of high-speed magic-angle spinning technology, the resolution of solid-state NMR spectroscopy was significantly improved. Theoretically, it surpasses the molecular weight limitations of liquid NMR, gradually being applied to the study of dynamic conformations of complex biomolecular systems.
However, the challenges of low signal strength and resolution remain, limiting its widespread application in solid-state biomolecular NMR research. Hydrogen and fluorine atoms, due to their high gyromagnetic ratios and strong NMR signals, emerge as ideal candidates for NMR observation.
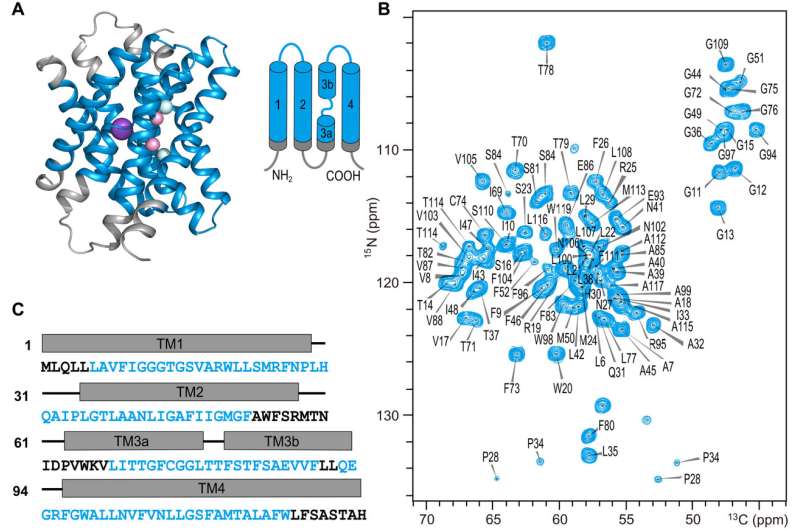
The Fluc-Ec1 protein, composed of about 130 amino acids, has a unique structure and demonstrates high selectivity towards fluoride ions. The researchers investigated the Fluc-Ec1 conformation in phospholipid bilayers and introduced 4-(trifluoromethyl)-l-phenylalanine (tfmF) into the vestibule space by the codon extension method.
Successful 19F-19F spin diffusion transfer from tfmF to the F− ions with −116.4-ppm chemical shift indicated F− ion binding (F0 sites) in the vestibules in high-F− ion solutions. The researchers detected water-protein interactions directly through a 1H-1H spin diffusion experiment in which magnetization from bound water could be transferred to the protein and determined the spin type is H2O rather than 19F at the F1 site.
The researchers explored its structure and functionality, proposing a new fluoride ion permeation model that provides a scientific basis for understanding the permeation and gating mechanisms in the Fluc channel.
More information: Jin Zhang et al, Fluoride permeation mechanism of the Fluc channel in liposomes revealed by solid-state NMR, Science Advances (2023). DOI: 10.1126/sciadv.adg9709
Journal information: Science Advances
Provided by University of Science and Technology of China