This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Iron-based molecular switch improves reaction yield by modulating zeolite catalyst acidity
A molecular switch, or molecule that changes in response to varying environmental stimuli, has successfully modified the acidity of a zeolite catalyst to improve the yield of paraxylene from methanol in heterogeneous catalysis, or a reaction where the catalyst, or molecule that facilitates a chemical reaction, and the reactants are in different phases, such as liquid and solid.
A recent study demonstrates that molecular switches can successfully modify the redox state, or charge, of moderately acidic reaction catalysts to improve catalyst activity, product selectivity and reaction yield.
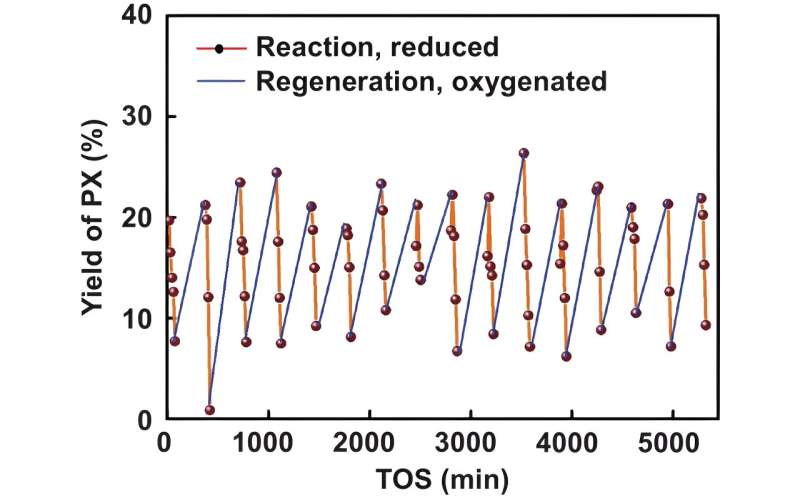
A team of chemical engineers recently improved the yield of paraxylene (PX) from methanol approximately three- to six-fold by employing an iron-based molecular redox switch designed to modify the acidity of a ZSM-5 (Zeolite Socony Mobil-5) heterogeneous catalyst. In this reaction, the researchers created PX via the methanol-to-aromatics (MTA) process while incorporating an iron-based redox switch to tune the acidity of the zeolite-based catalyst, reduce side reactions and improve overall reaction yield.
The team published the results of their study in the journal Carbon Future.
"A zeolite-based catalyst with [moderate] acidity is [paramount] to obtain[ing] [a] high yield of paraxylene from methanol," said Weizhong Qian, principal investigator of the study and professor at the Department of Chemical Engineering at Tsinghua University in Beijing, China and the Ordos Laboratory in Inner Mongolia, China.
"But pure reduction of the reaction (methanol to aromatics) resulted in [the gradual decrease] of acidity of the catalyst and the decrease of the yield of paraxylene. [R]edox switches are necessary to recover acidity," said Qian.
Qian's team chose the iron oxide redox molecular switch to finely tune the acidity of the zeolite-based catalyst based on their ability to easily modify reaction conditions: reducing conditions, for example, convert methanol to PX, whereas oxidative conditions regenerate the reaction catalyst. The redox state of the iron switch was controlled by hydrocarbons or H2 existed in the reaction condition and by adding air to the reaction to create oxidating conditions.
The team performed a total of 16 reaction-catalyst regeneration cycles to determine the stability of the molecular switch and the reaction efficiency. The products of each reaction were determined using gas chromatography at varying points during the reaction.
Ultimately, the iron-based redox switch demonstrated stability through 16 regeneration cycles over the course of 80 hours. The use of the molecular switch to tune catalyst acidity improved overall yield three- to six-times higher than previously reported MTA reactions and five- to 10-times higher than catalytic reforming of naphtha reactions, which is the primary means PX is produced today.
"Paraxylene remain[s]... the most important aromatic [compound in the preparation of] synthetic fibers, [such as PET, of which over 60 million tons are produced each year], for cloth[ing production]. But its preparation is of typically low efficiency, [requiring] heavy energy consumption [and] long-chain reaction and separation routes. [This] is highly undesirable in the era of carbon neutrality," said Qian.
While molecular switches have been frequently used in other fields, such as computer science and molecular biology, they are less often used in chemical engineering to regenerate catalysts. This research may advance the use of redox switches to improve the efficiency of other chemical reactions.
As a next step, the team wants to improve the stability of the reaction catalyst. Overall, the research team aspires to produce PX in the most time-, energy- and cost-efficient means possible. "The ultimate goal will be the direct production of high-purity and high-yield paraxylene from methanol, [without the requirement of the] aromatic complex, the most energy consumptive unit in the [process] of catalytic reforming of naphtha to produce paraxylene," said Qian.
More information: Qiongfang Hu et al, "Redox switches" of Fe species on zeolite catalysts: Modulating the acidity and the para-xylene yield from methanol, Carbon Future (2023). DOI: 10.26599/CF.2023.9200001 www.sciopen.com/article/10.26599/CF.2023.9200001
Provided by Tsinghua University Press