This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Zentropy and the art of creating new ferroelectric materials
Systems in the universe trend toward disorder, with only applied energy keeping the chaos at bay. The concept is called entropy, and examples can be found everywhere: ice melting, campfire burning, water boiling. Zentropy theory, however, adds another level to the mix.
A team led by Zi-Kui Liu, of the Dorothy Pate Enright Professor of Materials Science and Engineering at Penn State, developed the theory. The "Z" in zentropy stands for the German word Zustandssumm, meaning "sum over states" of entropy. Alternatively, Liu said, zentropy may be considered as a play on the term "zen" from Buddhism and entropy to gain insight on the nature of a system. The idea, Liu said, is to consider how entropy can occur over multiple scales within a system to help predict potential outcomes of the system when influenced by its surroundings.
Liu and his research team have published their latest paper on the concept, providing evidence that the approach may offer a way to predict the outcome of experiments and enable more efficient discovery and design of new ferroelectric materials. The work, which incorporates some intuition and a lot of physics to provide a parameter-free pathway to predicting how advanced materials behave, was published in Scripta Materialia.
Ferroelectrics have unique properties, making them valuable for a variety of applications both now and in developing materials, researchers said. One such property is spontaneous electric polarization that can be reversed by applying an electric field, which facilitate technologies ranging from ultrasounds to ink-jet printers to energy-efficient RAM for computers to the ferroelectric-driven gyroscope in smartphones that enable smooth videos and sharp photos.
To develop these technologies, researchers need to experiment to understand the behavior of such polarization and its reversal. For efficiency's sake, the researchers usually design their experiments based on predicted outcomes. Typically, such predictions require adjustments called "fitting parameters" to closely match real-world variables, which take time and energy to determine. But zentropy can integrate top-down statistical and bottom-up quantum mechanics to predict experimental measures of the system without such adjustments.
"Of course, at the end of the day, the experiments are the ultimate test, but we found that zentropy can provide a quantitative prediction that can narrow down the possibilities significantly," Liu said. "You can design better experiments to explore ferroelectric material and the research work can move much faster, and this means you save time, energy and money and are more efficient."
While Liu and his team have successfully applied zentropy theory to predict the magnetic properties of a range of materials for various phenomena, discovering how to apply it to ferroelectric materials has been tricky.
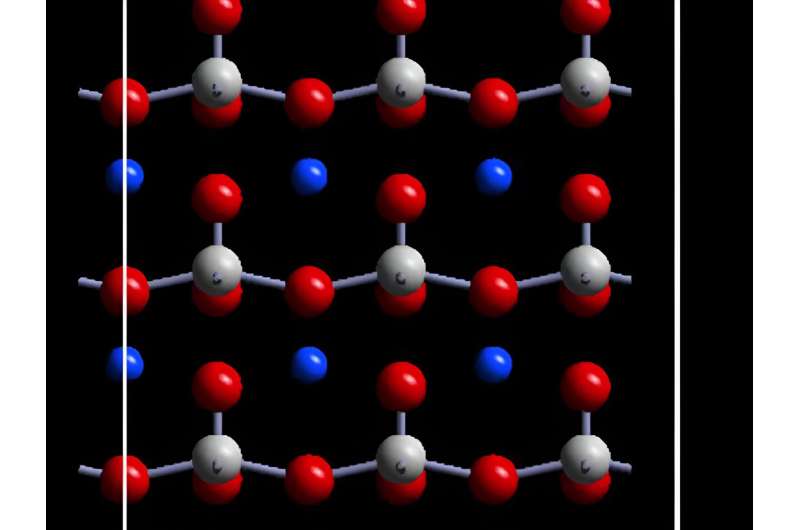
In the current study, the researchers reported finding a method to apply zentropy theory to ferroelectrics, focusing on lead titanate. Like all ferroelectrics, lead titanate possesses electric polarization that can be reversed when external electric fields, temperature changes or mechanical stress is applied.
As an electric field reverses, electric polarization reverses, the system transitions from ordered in one direction to disordered, and then to ordered again as the system settles into the new direction. However, this ferroelectricity occurs only below a critical temperature unique to each ferroelectric material.
Above this temperature, ferroelectricity—the ability to reverse polarization—disappears and paraelectricity—the ability to become polarized—emerges. The change is called the phase transition. The measurement of those temperatures can indicate critical information about the outcome of various experiments, Liu said. However, predicting the phase transition prior to an experiment is nearly impossible.
"No theory and method can accurately predict the free energy of the ferroelectric materials and the phase transitions prior to the experiments," Liu said. "The best prediction of transition temperature is more than 100° away from the experiment's actual temperature."
This discrepancy arises due to the unknown uncertainties in models, as well as fitting parameters that could not consider all salient information affecting the actual measurements. For example, an often-used theory characterizes macroscopic features of ferroelectricity and paraelectricity, but does not consider microscopic features such as dynamic domain walls—boundaries between regions with distinct polarization characteristics within the material. These configurations are building blocks of the system and fluctuate significantly with respect to temperature and electric field.
In ferroelectrics, the configuration of electric dipoles in the material can change the direction of polarization. The researchers applied zentropy to predict the phase transitions in lead titanate, including identifying three types of possible configurations in the material.
The predictions made by the researchers were effective and in agreement with observations made during experiments reported in the scientific literature, according to Liu. They used publicly available data on domain wall energies to predict a transition temperature of 776° Kelvin, showing a remarkable agreement with the observed experimental transition temperature of 763° Kelvin.
Liu said the team is working on further reducing the difference between predicted and observed temperatures with better predictions of domain wall energies as a function of temperature.
This ability to predict transition temperature so closely to the actual measurements can provide valuable insights into the physics of ferroelectric material—and help scientists to better their experimental designs, Liu said.
"This basically means you can have some intuitions and a predictive approach on how a material behaves both microscopically and macroscopically before you conduct the experiments," Liu said. "We can start predicting the outcome accurately before the experiment."
Along with Liu, other researchers in the study from Penn State include Shun-Li Shang, research professor of materials science and engineering; Yi Wang, research professor of materials science and engineering; and Jinglian Du, research fellow in materials science and engineering at the time of the study.
More information: Zi-Kui Liu et al, Parameter-free prediction of phase transition in PbTiO3 through combination of quantum mechanics and statistical mechanics, Scripta Materialia (2023). DOI: 10.1016/j.scriptamat.2023.115480
Journal information: Scripta Materialia
Provided by Pennsylvania State University