This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Serum amyloid A: Exploring links between the beneficial and pathologic actions of an enigmatic protein
Serum amyloid A (SAA) is a family of ancient proteins that can be traced from present-day humans back half a billion years to sea cucumbers and oysters. A new study by researchers from the Boston University Chobanian & Avedisian School of Medicine explores the link between the dual nature of this small plasma protein: how it works to clear toxic debris from wounds and inflammation sites, but also its role in forming fibrous deposits of the pathologic amyloid in vital organs such as the kidney and liver in the life-threatening disease AA amyloidosis.
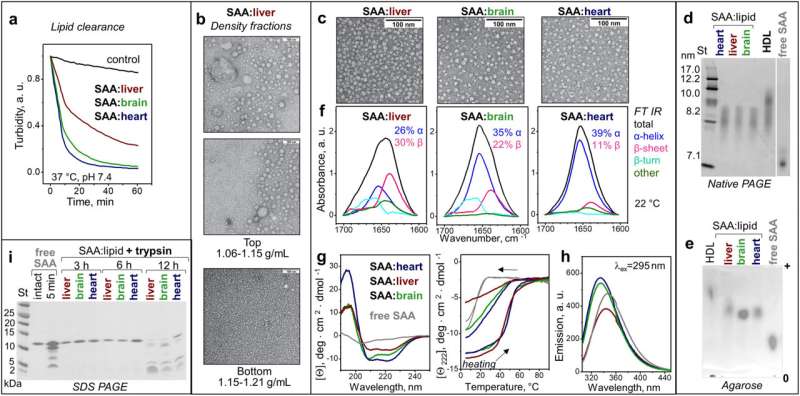
"This work demonstrated that SAA acts as a universal protein detergent more potent than bile," said Shobini Jayaraman, Ph.D., senior staff scientist in pharmacology, physiology & biophysics and the lead author of this study.
Jayaraman demonstrated that SAA rapidly makes lipids soluble, converting them into nanoparticles that can be further broken down by enzymes and carried away by cells.
"This finding solidifies our theory that SAA clears cell membrane debris from the sites of injury and inflammation in animals and humans," said Olga Gursky, Ph.D., professor of pharmacology, physiology & biophysics, the senior author of the study and the principal investigator on the NIH grant that supports this research.
Amyloidosis occurs when the structure of proteins in the body changes and forms clumps of fibrils on organs and tissues. AA amyloidosis is a major complication when SAA levels in blood rise in response to long-lasting infection or chronic inflammation. The BU researchers posited that the stable binding of SAA to lipid nanoparticles prevents the formation of pathologic amyloids, but lipases (enzymes that help in fat digestion and membrane clearance) can compromise that binding.
The study found that secretory phospholipase A2, an inflammatory enzyme that raises in blood together with SAA and acts synergistically with it in removing cell membrane debris, can also promote SAA amyloid deposition, said Jayaraman.
The researchers used recombinant murine or human SAA. They tested for solubility and nanoparticle formation and used protein spectroscopy, electron microscopy, chromatography and biochemical assays to analyze the various complexes produced under lab conditions that mimicked what occurs in the body.
The dual nature of proteins that both transport lipids and form amyloids is present in other diseases, like Alzheimer's amyloid-beta peptide. Apolipoprotein A-I, the major protein in "good cholesterol," can also form pathologic amyloids resulting in atherosclerosis and systemic amyloidosis, said Gursky.
"We hope that our study will help to better understand and treat AA amyloidosis. Key results can be extrapolated to other amyloid diseases, which are life-threatening incurable diseases affecting millions of patients worldwide," researchers said.
These findings appear online in the Journal of Lipid Research. Other BU researchers who worked on the study included Angela Urdaneta, Ph.D., and Esther Bullitt, Ph.D. Marcus Fändrich, director of the Institute of Protein Biochemistry at Ulm University in Germany, also worked on the study.
More information: Shobini Jayaraman et al, Lipid Clearance and Amyloid Formation by Serum Amyloid A: Exploring the Links Between Beneficial and Pathologic Actions of an Enigmatic Protein, Journal of Lipid Research (2023). DOI: 10.1016/j.jlr.2023.100429
Journal information: Journal of Lipid Research
Provided by Boston University School of Medicine