This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Nobel-winning bodily 'pressure sensors' filmed for first time
Imperial researchers have filmed, for the first time, the activity of bodily "pressure sensors" whose discoverers won the 2021 Nobel Prize in Physiology or Medicine.
The sensors—ion channels called Piezo1 and Piezo2—are found throughout the body, from the heart, bladder and kidneys to the immune and nervous systems. Imperial College London researchers have now imaged them for the first time, potentially lighting the way for new drug targets in a range of diseases.
Responsible for detecting and responding to changes in pressure, Piezo channels play a crucial role in regulating blood pressure, respiration, bladder control, and the immune system. The channels could therefore be important future drug targets for diseases including cancer.
Until now, investigating Piezo channel activity could only be done by invasive or indirect techniques, such as monitoring general calcium fluctuations in cells.
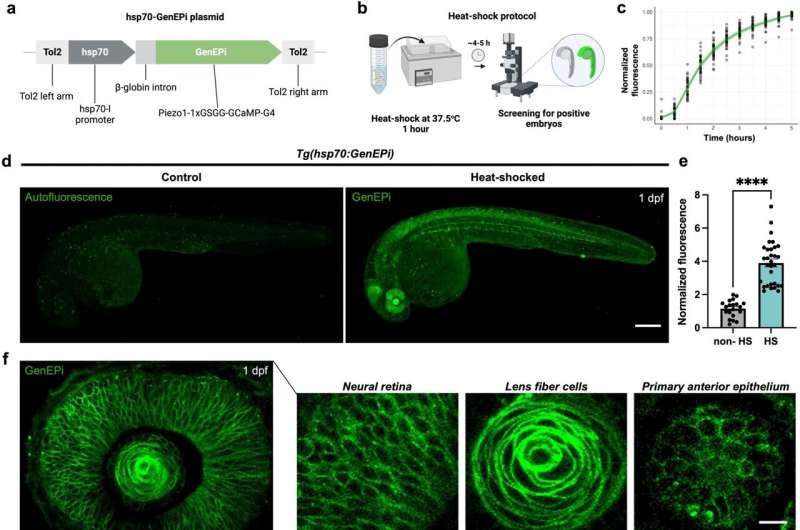
To image the channels, Imperial researchers led by Dr. Periklis Pantazis developed a highly specific biosensor, called GenEPi, which lights up under a microscope when Piezo1 channels are activated. They tested GenEPi at both the cellular and whole-organism level, successfully highlighting Piezo1 activity in human kidney, foreskin, and cervical cancer cells, as well as beating mouse heart cells, and whole zebrafish embryos.
This is the first time a non-invasive and specific visual method for studying Piezo1 activity has been developed, allowing researchers to capture its behavior in detail during healthy and disease states. The work is published in Nature Communications.
Co-lead author Konstantinos Kalyviotis, Ph.D. researcher at Imperial's Department of Bioengineering, who co-led the work with Sine Yaganoglu, former Ph.D. researcher in the Pantazis Lab at ETH Zurich, said, "The pressure-responsive Piezo channels are extremely important for maintaining the smooth operation of our bodies. They play a key role in sensing forces within us and regulating processes such as blood pressure, as well as prompting us to use the restroom when our bladder is stretched and full."
"Piezo1 is involved in many systems that are vital for life. Our visualizations could be instrumental in revealing the full power of Piezo1 activity in different cellular contexts."
New drug targets?
To study the channels, Imperial and ETH Zurich researchers engineered GenEPi, a calcium ion reporter that links specifically to the Piezo1 channel without affecting its function. GenEPi glows brightly only when the channels open and allow calcium ions to pass through. Hence scientists can now, for the first time, see when and where they are active.
The ability to visualize the channels in action could lead to a better understanding of their role in fundamental physiological processes, such as new blood vessel formation, cell migration and cell proliferation—processes that are exploited by cancer cells during tumor growth and metastasis.
Once the activity of the channels in disease is better understood, they could be targets for a new non-chemical drug type that works mechanically to complement more widely-used drugs.
Senior author Dr. Periklis Pantazis, also of the Department of Bioengineering, said, "Being able to see Piezo1 activity will allow researchers to visualize the effects of non-chemical drugs on Piezo1-dependent diseases. I would love to see drugs based on this mechanism developed in the next ten years."
Glowing and sensing
The concept of Piezo ion channels was initially hinted at in 2010, when the laboratory of Ardem Patapoutian identified cells producing measurable electric signals when poked with a micropipette. This initial observation eventually led to the discovery of a novel class of pressure-responsive channels, aptly named Piezo after the Greek word "πίεσις/πιέζω," signifying pressure. This breakthrough won Patapoutian the 2021 Nobel Prize in Physiology or Medicine.
The Imperial researchers focused on Piezo1, which is found in almost every cell in our bodies and helps regulating growth, optimal functioning in response to the environmental forces, and disease.
Mechanical stimuli, such as changes in pressure, cause Piezo channels to open, which lets mainly calcium ions through. This influx of ions triggers a response which reaches the brain and allows it to respond—for example by sending conscious signals that the bladder is full, or subconscious signals that blood pressure is high. These messages induce the body to respond and restore order—a process called homeostasis.
The researchers are now applying the design principle of GenEPi to developing and engineering optical reporters of other ion channels without affecting their function. They will also continue to investigate Piezo1's role in a range of diseases, including cancer.
Konstantinos said, "We stand at the dawn of a journey to unveil the profound impact of Piezo channels on health and disease. Our innovative biosensor stands as a powerful tool that holds the promise of illuminating this path of discovery, driving us onward in our continuing pursuit of knowledge."
More information: Sine Yaganoglu et al, Highly specific and non-invasive imaging of Piezo1-dependent activity across scales using GenEPi, Nature Communications (2023). DOI: 10.1038/s41467-023-40134-y
Provided by Imperial College London