This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Simultaneous synthesis and fixing of covalent organic frameworks
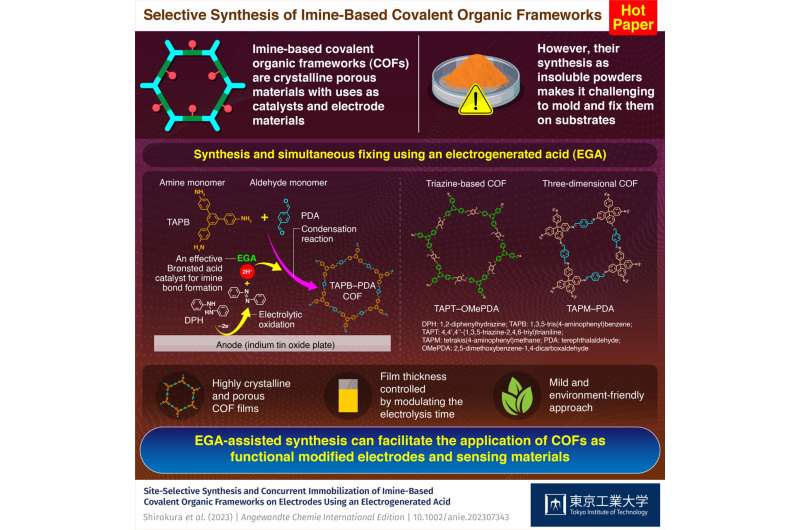
Covalent organic frameworks (COFs) are versatile materials composed of interconnected organic molecules held together by covalent bonds. These frameworks can be constructed in two-dimensional or three-dimensional (3D) forms which possess a unique combination of low density, high surface area, and easily tunable properties. Among the various types of COFs, imine-linked COFs have garnered considerable attention owing to their exceptional thermal and chemical stability as well as their broad scope of monomeric starting materials.
However, traditional bulk synthetic methods for COFs often yield powders that are insoluble in common organic solvents, posing challenges during their subsequent molding and fixing on substrates. While alternative fabrication approaches, such as exfoliation of bulk COFs into nanosheets, employment of novel interfaces, and use of templates for freestanding films overcome this limitation, they usually involve multiple steps and produce low-quality COF structures.
Recently, a team of researchers from Japan, led by Professor Shinsuke Inagi from Tokyo Institute of Technology (Tokyo Tech), has developed a novel method for synthesizing and fixing high-quality imine-based COFs. Their work was published in Angewandte Chemie International Edition.
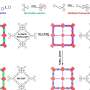
"The proposed method utilizes an electrogenerated acid (EGA), produced via electrochemical oxidation of 1,2-diphenylhydrazine (DPH) in an organic electrolytic solution, as a catalyst for COF synthesis and immobilization onto electrode surfaces. It acts as a strong Brønsted acid, promoting the condensation reaction between amine and aldehyde monomers—the building blocks of imine-based COFs—to form covalent bond networks," explains Prof. Inagi.
The team chose DPH as the EGA source due to its low oxidation potential and acid-releasing properties and used 1,3,5-tris(4-aminophenyl)benzene (TAPB) and terephthalaldehyde (PDA). By employing the potential-sweep method for electrolysis, they successfully obtained film-like COF deposits on indium tin oxide electrodes immersed in nitromethane. The TAPB–PDA COF films possessed high crystallinity and porosity. In addition, their thickness could be controlled by modulating the electrolysis time.
The researchers also extended their electrochemistry-based approach to the synthesis of other structures, including triazine-based and 3D COFs.
In conclusion, Prof. Inagi highlights the future potential of the proposed method. "It eliminates the need for long reaction times, high temperatures, and Lewis acid catalysts typically required in conventional COF synthesis, making it environment friendly," he explains. "Moreover, the direct fixing of COF films onto electrodes is promising for COF-based applications, especially in functional modified electrodes and sensing materials."
More information: Tomoki Shirokura et al, Site‐Selective Synthesis and Concurrent Immobilization of Imine‐Based Covalent Organic Frameworks on Electrodes Using an Electrogenerated Acid, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202307343
Journal information: Angewandte Chemie International Edition
Provided by Tokyo Institute of Technology