This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Understanding the regulation of apicoplast gene expression in the malaria parasite
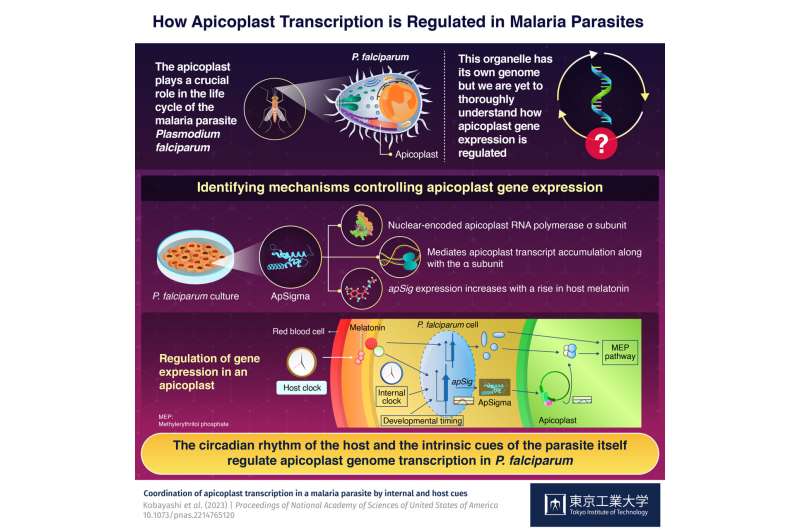
Gene expression within the apicoplast, an organelle in the malaria parasite Plasmodium falciparum, is regulated by melatonin (the circadian signaling hormone) in host blood and intrinsic parasite cues, via a factor called ApSigma, as identified by a recent study aided by Tokyo Tech's World Research Hub Initiative. The regulatory system highlighted in this study might be a future target for malaria treatment.
Malaria is one of the biggest public health risks, with about 240 million people from across the globe contracting it every year. However, this life-threatening disease is not contagious. It is transmitted by the bite of a female Anopheles mosquito infected with the malaria parasite, Plasmodium falciparum. This parasite enters the human body through the mosquito bite and causes symptoms like fever, cold, fatigue, and headache, which are highly periodic. The periodicity of symptoms can be linked to the synchronization of the parasite's life cycle with the circadian rhythm—i.e., the 24-hour internal biological clock—of the infected person or the host.
P. falciparum contains an apicoplast, a unique cellular organelle which contains its own genome and is crucial for the parasite's life cycle. Despite its importance, however, not much is known about the mechanisms regulating gene expression in apicoplasts and their potential role in modulating the observed periodicity of malaria symptoms, or the life cycle of P. falciparum.
This is why a team of scientists led by Professor Kan Tanaka, of Tokyo Institute of Technology (Tokyo Tech), undertook a joint research project to take a closer look at the underlying mechanisms that mediate apicoplast gene expression. The work, published in Proceedings of the National Academy of Sciences (PNAS), was a result of collaboration with co-authors Professor Kiyoshi Kita of Nagasaki University and Professor Antony N. Dodd, a group leader at the John Innes Center in the UK—also a visiting professor at Tokyo Tech.
"Previous studies have shown that certain plant σ subunits participate in the circadian regulation of gene expression in plastids (i.e., organelles like the apicoplast). Therefore, the present study hypothesized that a nuclear-encoded σ subunit might coordinate apicoplast gene expression with the life cycle of P. falciparum or the circadian rhythm of its host," explains Prof. Tanaka.
The team cultured P. falciparum in a lab and studied it using phylogenetic analysis and immunofluorescence microscopy techniques. As a result, they identified ApSigma, a nuclear encoded apicoplast RNA polymerase σ subunit. It, along with the α subunit, likely mediates apicoplast transcript accumulation, whose periodicity is akin to that of the parasite's developmental control. In addition, apicoplast transcription and expression of the apicoplast subunit gene, apSig, increased in the presence of melatonin, the circadian signaling hormone present in host blood.
Based on the data collected from different tests, the scientists suggest that there is an evolutionarily preserved regulatory system in which the host's circadian rhythm is integrated with the parasite's intrinsic cues. Together, they coordinate genome transcription in the apicoplast of P. falciparum. This work forms a solid basis for further studies in the field aiming to comprehensively explain the regulatory mechanisms of Plasmodium's cell cycle.
In conclusion, Prof. Tanaka highlights the future implications of the present research. "Malaria kills hundreds of thousands of people across the world, every year. This study identifies a regulatory system that might be a future target for malaria treatment."
Professor Dodd, adds, "It is amazing that a process we identified in plants has led to the discovery of an equivalent mechanism in a globally important pathogen. The new protein and mechanism identified could present a new target for the development of drugs for the treatment and or prevention of malaria, in both humans and farm animals."
Professor Kita states, "This research demonstrates the value of international and interdisciplinary collaboration, and the power of plant sciences and microbiology to drive unusual and novel discoveries that could be of considerable global benefit," he says.
More information: Kobayashi, Yuki et al, Coordination of apicoplast transcription in a malaria parasite by internal and host cues, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2214765120
Journal information: Proceedings of the National Academy of Sciences
Provided by Tokyo Institute of Technology