This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers remove protein aggregates from cells
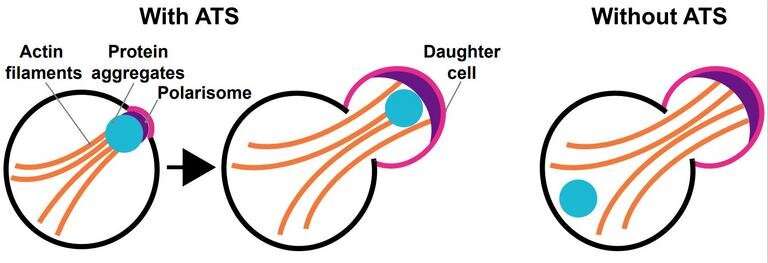
Protein aggregates accumulate during aging and are linked to neurodegenerative diseases like Alzheimer's, Parkinson's or Huntington's disease. A new study by the Nyström lab at Gothenburg University, in collaboration with the Max Planck Institute for Biology of Aging in Germany, describes a novel, engineered approach that makes protein aggregates amenable to spatial manipulations in both budding yeast and human cells.
Many neurodegenerative diseases like Alzheimer's, Parkinson's or Huntington's disease are associated with the aggregation of misfolded proteins but whether or not these aggregates contribute to these diseases is not clear.
The research group of Professor Thomas Nyström from Gothenburg University was able to export such protein aggregates from cells in an engineered manner. The system was first developed in the widely used model organism, budding yeast, but was extended also for use in human cells. The study is published in the journal Nature Communications.
Free of protein aggregates
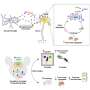
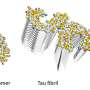
The authors achieved this synthetic cellular export system by fusing an aggregate-binding protein to a daughter-cell-targeting factor such that when the daughter cell is pinched off, the mother cell is free of protein aggregates. This approach was effective in dealing with endogenous, age-associated protein aggregates, as well as with mutant Huntingtin aggregates associated with the Huntington's disease.
Using this daughter targeting system, they showed that the export of mutant Huntingtin protected yeast mother cells from cell death suggesting that large Huntingtin aggregates may be highly toxic and contribute to the disease, a trait that has been widely debated.
Potential future therapy
"To our knowledge this is the first demonstration that protein aggregates can be exported from cells in a controlled, engineered manner. Other kinds of cellular damage might be also exported from cells with adapted versions of our targeting system," says Dr. Arthur Fischbach, postdoc and the lead author of the study.
"Although we currently lack supporting data, there is a possibility that the ATS concept could be employed in the future as a potential novel therapeutic approach for neurodegenerative diseases or at least for gaining a better understanding of them. Given the urgency, there is a strong demand for innovative therapies in this area," he concludes.
More information: Arthur Fischbach et al, Artificial Hsp104-mediated systems for re-localizing protein aggregates, Nature Communications (2023). DOI: 10.1038/s41467-023-37706-3
Journal information: Nature Communications
Provided by University of Gothenburg