This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Activated partial metal sites in high entropy oxides for enhanced catalytic performance
High entropy oxides (HEOs) have been tentatively and prospectively applied for catalysis and energy storage. However, it is hard to further enhance their performance due to the difficult regulation of HEOs' physical-chemical properties. Although some optimized strategies, such as the introduction of noble metal, have improved the properties and performance of HEOs in a simple and effective way, current methods do not lead to commercial preparations or industrial application.
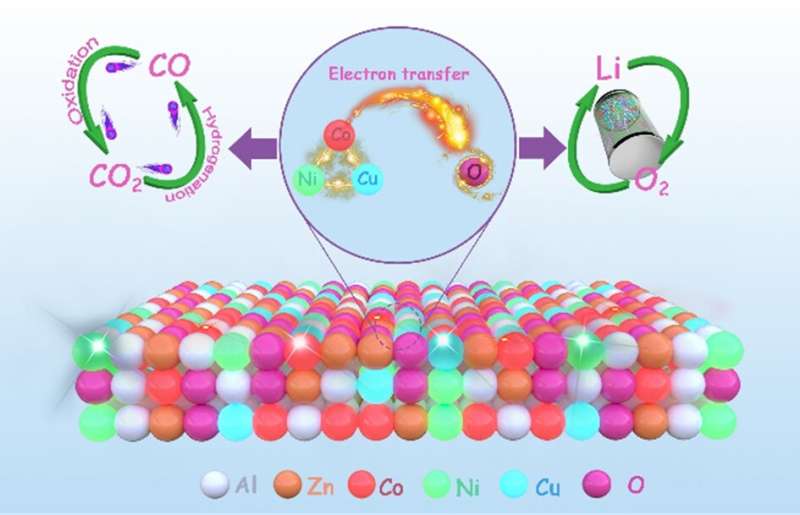
Recently, a research team led by Prof. Zhong-Shuai Wu from State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, reported a general in-situ modulation strategy of solid phase combustion using a thiourea addition and alkali liquor treatment to activate metal sites and lattice oxygen species of CuCoNiZnAl HEOs.
Consequently, the activated HEOs not only display higher CO2 hydrogenation and CO oxidation activity, but also have better electrocatalytic activity (discharge/charge capacities of 12,049/9,901 mAh/g) with excellent cycle stability (2,500 h) on an Li-O2 battery than that of pristine HEOs. The results were published in Chinese Journal of Catalysis.
The optimized HEOs using the thiourea addition (CuCoNiZnAl-T) and alkali liquor treatment (CuCoNiZnAl-T-NaOH) exhibit a similar crystal structure to that of CuCoNiZnAl, but higher BET surface area. Meanwhile, the reducibility of the CuCoNiZnAl-T-NaOH catalyst is much better than CuCoNiZnAl-T and CuCoNiZnAl. In addition, CuCoNiZnAl and CuCoNiZnAl-T present irregular massive morphology, whereas CuCoNiZnAl-T-NaOH shows a sheet-like morphology. The CuCoNiZnAl-T-NaOH also possessed more cationic vacancies, distorted lattice and more active lattice oxygen species.
As a result, CuCoNiZnAl-T-NaOH not only shows higher CO2 conversion than that of CuCoNiZnAl and CuCoNiZnAl-T in the temperature range from 350°C to 600°C, but also exhibits higher CO conversion than those of CuCoNiZnAl and CuCoNiZnAl-T in the whole temperature range (especially between 160 and 220 °C). More importantly, the CuCoNiZnAl-T-NaOH cathode delivers much higher discharge/charge capacities of 12,049/9,901 mAh/g than those of CuCoNiZnAl-T (11,917/8,071 mAh/g) and CuCoNiZnAl (7,260/5,224 mAh/g) with a stable stability (~2,500 h).
The excellent performance is mainly attributed to the easier electron transfer between Cu/Ni/Co sites and lattice oxygen species in the framework of CuCoNiZnAl-T-NaOH. Therefore, this present work offers a novel manner of optimizing HEOs with targeted activated metal sites as highly active heterogeneous thermal and electrochemical catalysts for redox reactions and energy storage in an environmentally friendly and cost-effective way.
More information: Jinxing Mi et al, Activation of partial metal sites in high-entropy oxides for enhancing thermal and electrochemical catalysis, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64409-2
Provided by Chinese Academy of Sciences