This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Enhanced oxygen reduction reaction activity and biperiodic trends of lanthanide-doped molybdenum disulfide
A research group led by Prof. Wang Liping at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences has reported the enhanced oxygen reduction reaction (ORR) activity and biperiodic chemical trends of lanthanide-doped molybdenum disulfide (Ln-MoS2). The study was published in Nature Communications.
MoS2 has broad application prospects in catalysis, solid lubrication, optoelectronics, and other fields. Various lanthanides (Ln), such as Sm, Eu, Dy, Ho, Er, and Yb, etc., can be doped into MoS2 to modify its physicochemical properties.
By reducing O2 to H2O, surface oxygen reduction plays a critical role in the performance and lifetime of Ln-MoS2-based functional materials, coatings and devices, such as the fuel cell efficiency and device galvanic corrosion. Exploring the ORR activity on the surface of Ln-MoS2 and its orbital chemistry mechanism can provide guidance for the practical application design, precise performance regulation and effective protection of Ln-MoS2 systems.
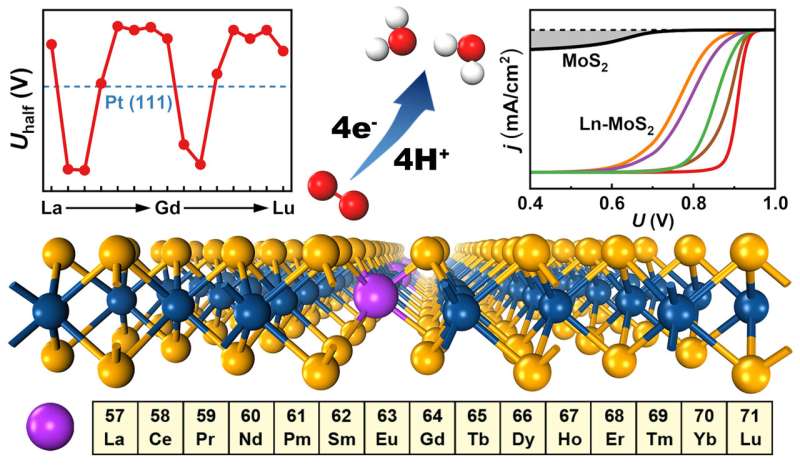
Through density-functional theory calculations, the researchers investigated the ORR process on all the 15 Ln-MoS2 (Ln = La ~ Lu) surfaces.
The doping of Ln significantly enhanced the ORR activity on Ln-MoS2 surfaces. In addition, a fascinating modulating biperiodic chemical trend of the ORR activity was observed.
Moreover, the water effect on the loose f/liquid interface was accurately simulated based on the thermodynamic statistics. Current-potential polarization curve simulations were also performed to quantitatively reveal the ORR activity and effectively guide the related experiments.
In-depth electronic structure analysis revealed that the enhancement of ORR activity can be attributed to a defect-state pairing mechanism, which selectively stabilizes the hydroxyl and hydroperoxyl adsorbates on Ln-MoS2, thus significantly lowering the ORR energy barrier.
Furthermore, a generic orbital chemistry mechanism of Ln-MoS2 systems was proposed, which helps to explain the intrinsic biperiodic trends observed in various electronic, thermodynamic, and kinetic properties.
This work sheds light on the design of superior Ln-MoS2-based materials and related systems with promising application and commercial prospects in electrocatalysts, optoelectronic nanodevices, and anti-corrosion coatings.
More information: Yu Hao et al, Lanthanide-doped MoS2 with enhanced oxygen reduction activity and biperiodic chemical trends, Nature Communications (2023). DOI: 10.1038/s41467-023-39100-5
Journal information: Nature Communications
Provided by Chinese Academy of Sciences