This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Homing in on effective separation of radionuclides to fuel a circular economy
Nuclear scientists are on a mission to help create a circular economy where "waste" is a useful resource. Used nuclear fuel can be recycled, and scientists are working to understand how radiation affects this recycling process. Researchers hope to improve the recycling process and extract more useful chemical elements from the fuel.
The nuclear industry has one challenge seldom faced by other industries: radiation. A collaboration between the United Kingdom's National Nuclear Laboratory (NNL), Idaho National Laboratory (INL) in the United States and The University of Manchester in the U.K. has shed light on how radiation affects one very important molecule, acetohydroxamic acid (AHA). This work was featured on the cover of the journal ChemPhysChem.
Dr. Dan Whittaker leads the Advanced Recycle and Isotope Separations theme for the NNL, "The effect of radiation on the solvents used in separating useful isotopes from dissolved nuclear fuel is often considered to be quite small. We know that other chemical reactions like hydrolysis are important, but we need to really understand the radiation chemistry if we want to work towards more efficient extraction techniques. It's helpful to know that radiation doesn't have a big effect, but we need to test each new chemical so that we can make sure that advanced recycling techniques are developed correctly."
Building on pioneering work
Used nuclear fuel contains many chemical elements, some of which can be extracted and reused to make new nuclear fuel or used in other applications. For example, in health care, radioisotopes are used to treat medical conditions, while in space exploration, radioisotopes power missions, rovers and probes.
Traditional nuclear fuel recycling techniques were developed over half a century ago when the nuclear industry was in its infancy. The technology and knowledge have evolved significantly since those early days. In the not-too-distant future, this old technology can be replaced with a more efficient recycling process that uses skills nuclear scientists have honed over decades.
Developing advanced recycling technology
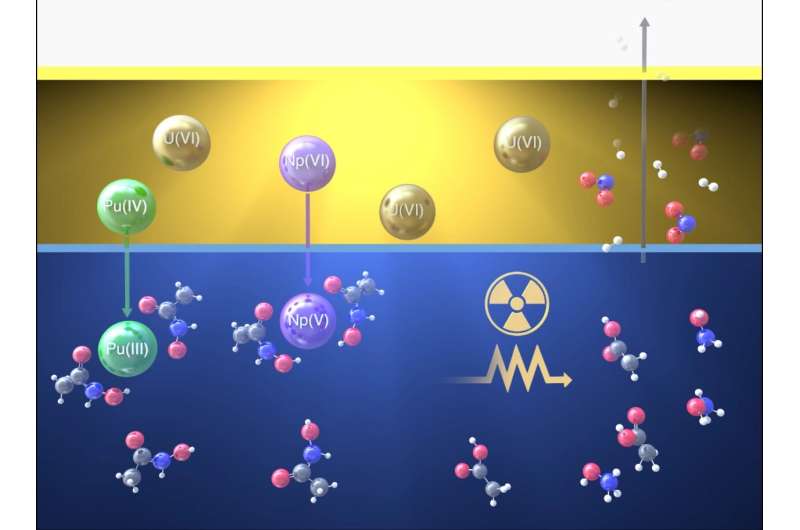
In traditional nuclear recycling techniques, used fuel is dissolved in nitric acid, turning it into a liquid. Selected metals are then extracted from the acidic solution using a chemical. The process works in a similar way to soap, where a chemical known as a surfactant clings to certain metals, pulling them out of a watery liquid and into an oily one. Other chemicals and metals are left behind in the water. Ideally, only specific metals like uranium would be extracted to make new fuel but other, less useful, metals are often extracted at the same time requiring additional purification.
In advanced nuclear recycling technologies additional chemicals are tested to improve this recycling process. One chemical of interest is AHA, or alpha hydroxy acids, which is more commonly used in medicine to treat bladder infections. This chemical can prevent neptunium and plutonium from being extracted along with the uranium, which means fewer purification steps are required to make new fuel, improving the efficiency of the process. Using AHA in the extraction process also contributes to international nonproliferation agreements by providing greater control of these metals.
Ultrafast chemical reactions caused by radiation
Predicting how radiation affects even simple chemicals like water and nitric acid is a challenge. Radiation causes hundreds of chemical reactions in a fraction of a second, which makes it hard to detecting them and build an accurate predictive model. New research led by INL's Dr. Jacy Conrad has developed a model for AHA that accurately predicts these myriad reactions. This model tells us how effective the chemical will be in improving the fuel recycling process.
To build the model, which relies on computing all the chemical reactions, some reactions had to be measured for the first time. A special technique provided a snapshot of the ultrafast chemical reactions caused by the absorption of radiation. In collaboration with Brookhaven National Laboratory's Laser Electron Accelerator Facility in the United States, incredibly quick, intense bursts of high energy electrons were used to emulate beta and gamma radiation effects.
Although fragments of irradiated AHA might continue to react, the ~30 picosecond pulse of the electron beam is timed to coincide with rapid analytical techniques to capture the behavior of short-lived chemicals in the solution. Using expert chemical knowledge of the reactions that produce these short-lived chemicals, garnered over decades from the wider radiation chemistry community, reaction rates can also be measured.
All possible reactions—almost 200 of them—along with their reaction rates, were used to build the predictive model. To ensure the model was accurate, more radiation work was carried out at INL's Center for Radiation Chemistry Research and the UoM's Dalton Cumbrian Facility. Using gamma radiation sources, researchers followed the progression of the chemical reactions over longer periods of time. The final stable products from these chemical reactions were identified. Their measured concentrations matched the values calculated by the model, proving that the model's predictions were accurate.
Looking ahead
Dr. Conrad developed the predictive model at INL and conducted the experiments, "The challenge of unraveling all the individual chemical reactions that contribute to the degradation of a single species is really exciting. It's a truly iterative process of comparing the model outputs to the steady-state gamma irradiations results, and then realizing there's another reaction that needs to be measured and repeating. At the end of the day, it's incredible to watch all the puzzle pieces come together, and better yet that this work can have such important impacts in optimizing used nuclear fuel reprocessing cycles."
Combining experiment with modeling showed that the effects of radiation under these very specific conditions are quite small; the bigger effect is caused by the acidic solution, which causes hydrolysis of the AHA. Investigations have focused on the interaction between AHA, nitric acid and radiation. The next step is to consider what will happen when a key component of the used nuclear fuel—neptunium—is included. Even this seemingly small change could have a big influence on the reactions induced by radiation. Work is underway to investigate this new twist, building on previous research.
More information: Jacy K. Conrad et al, Gamma Radiation‐Induced Degradation of Acetohydroxamic Acid (AHA) in Aqueous Nitrate and Nitric Acid Solutions Evaluated by Multiscale Modelling, ChemPhysChem (2022). DOI: 10.1002/cphc.202200749
Journal information: ChemPhysChem
Provided by National Nuclear Laboratory